filmov
tv
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)

Показать описание
The carbon atoms of C2H2 are sp hybridized. This makes it a challenge to draw, but I will show you the strategies in the video.
In particular, you need to show hybridized sp orbitals along the horizontal bond axis... and then you need unhybridized p orbital going up-and-down from each of the carbon atoms ... and then ANOTHER unhybridized p orbital drawn at an angle, to mimic the fact that it's in a third dimension, coming out of the page at you.
In particular, you need to show hybridized sp orbitals along the horizontal bond axis... and then you need unhybridized p orbital going up-and-down from each of the carbon atoms ... and then ANOTHER unhybridized p orbital drawn at an angle, to mimic the fact that it's in a third dimension, coming out of the page at you.
Orbital Overlap Diagram of Ethyne - Acetylene
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
Draw the Orbital Overlap Diagram for NH3 (ammonia)
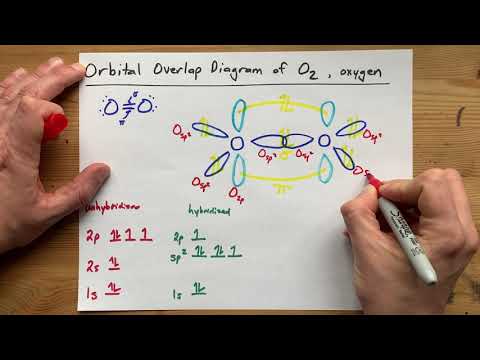
Draw the Orbital Overlap Diagram of O2 (Oxygen gas)
Draw the Orbital Overlap Diagram for HCl (Hydrochloric Acid)
Orbital Overlap Diagram for N2
Orbital Overlap Diagram of CO2 (carbon dioxide)
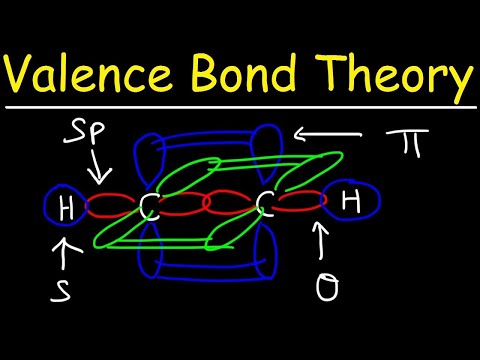
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
“DRAW THE ORBITAL DIAGRAM” Practice Problem || Organic Chemistry
Drawing Orbital Overlap Diagrams for Carbanions and Carbocations -Help Me With Organic Chemistry!
Steps in Drawing Orbital Overlapping & How to Draw Orbital Overlapping?
Drawing Molecular Orbital Pictures
Orbital Overlap Diagram of CO (carbon monoxide)
Valence Bond Theory & Hybrid Atomic Orbitals
Draw the orbital overlap diagram showing all bonds and lone pairs Label each of the bond in the mol
Drawing Molecular Orbital Diagrams
Orbital overlap diagram of Ethanoic Acid (Acetic acid)
drawing a bonding orbital picture for hydrogen cyanide
4.3 Orbital Overlap and Hybridisation (sp3d hybrid)
Orbital Overlap Diagram for NH3 (Ammonia)
Hybridisation of CO2
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry
Комментарии
 0:06:20
0:06:20
 0:10:06
0:10:06
 0:06:42
0:06:42
 0:07:26
0:07:26
 0:07:07
0:07:07
 0:07:59
0:07:59
 0:04:21
0:04:21
 0:09:18
0:09:18
 0:06:54
0:06:54
 0:07:54
0:07:54
 0:07:07
0:07:07
 0:03:59
0:03:59
 0:29:59
0:29:59
 0:08:58
0:08:58
 0:06:27
0:06:27
 0:10:39
0:10:39
 0:04:44
0:04:44
 0:11:05
0:11:05
 0:08:15
0:08:15
 0:03:33
0:03:33
 0:17:06
0:17:06
 0:06:13
0:06:13
 0:04:55
0:04:55
 0:06:17
0:06:17