filmov
tv
Orbital overlap diagram of Ethanoic Acid (Acetic acid)

Показать описание
sp2 hybridized orbitals overlapping to make the SIGMA bond between C and O
leftover p orbitals overlapping above-and-below to make the PI bond
Everything else is sigma, and the hybridizations are sp3... except for Hydrogen, which doesn't hybridize
leftover p orbitals overlapping above-and-below to make the PI bond
Everything else is sigma, and the hybridizations are sp3... except for Hydrogen, which doesn't hybridize
Orbital overlap diagram of Ethanoic Acid (Acetic acid)
Orbital Overlap Diagram of Ethyne - Acetylene
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
Hybridization, Hybrid Orbitals, and Bonding Diagrams
Drawing Orbital Overlap Diagrams for Carbanions and Carbocations -Help Me With Organic Chemistry!
Orbital Overlap Diagram for NH3 (Ammonia)
Draw the Orbital Overlap Diagram for NH3 (ammonia)
Orbital Overlap Diagram of CO2 (carbon dioxide)
Video 7 Orbital Overlap Diagrams
Orbital Overlap Diagram of CO (carbon monoxide)
Orbital Overlap Diagram for N2
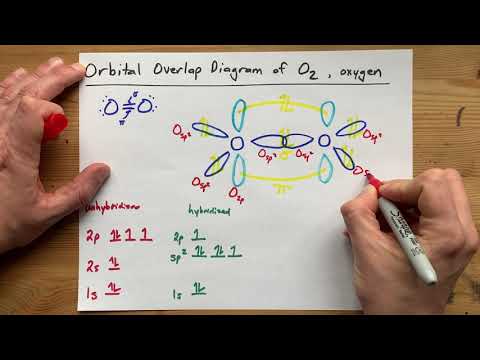
Draw the Orbital Overlap Diagram of O2 (Oxygen gas)
CHEM110: Hybrid Orbitals Part 3
Drawing Molecular Orbital Pictures
EASY Method to Find the Hybridization of an Atom | Chemistry |
Chemistry Part 34: More Realistic Molecular Shapes, Plus Hybrid Orbitals
“DRAW THE ORBITAL DIAGRAM” Practice Problem || Organic Chemistry
32 - Hybridization
Hybridisation of N2
Orbital overlapping HCOOH
Acids & Bases - Inductive Effect, Electronegativity, Hybridization, Resonance & Atomic Size
orbital picture of hydrogen cyanide
Hybridization of CH2CH2
Комментарии
 0:08:15
0:08:15
 0:06:20
0:06:20
 0:10:06
0:10:06
 0:06:42
0:06:42
 0:14:14
0:14:14
 0:03:59
0:03:59
 0:06:13
0:06:13
 0:07:07
0:07:07
 0:06:54
0:06:54
 0:20:55
0:20:55
 0:06:27
0:06:27
 0:09:18
0:09:18
 0:07:59
0:07:59
 0:21:05
0:21:05
 0:08:58
0:08:58
 0:04:08
0:04:08
 0:10:53
0:10:53
 0:07:07
0:07:07
 0:06:18
0:06:18
 0:04:00
0:04:00
 0:05:08
0:05:08
 0:09:19
0:09:19
 0:03:06
0:03:06
 0:07:30
0:07:30