filmov
tv
Orbital Overlap Diagram of CO2 (carbon dioxide)

Показать описание
The carbon atom is double bonded to EACH of the two oxygens.
You'll need to show an UNHYBRIDIZED 2p orbital in an up-down direction (I label it 2py here) and ANOTHER going into-and-out-of-the-page (I label is 2pz here). ONE of the oxygens will make a pi bond with the 2py orbital and the OTHER oxygen will make a pi bond with the 2pz orbital. Sigma bonds are shown along the bond axis (end to end overlap)
You'll need to show an UNHYBRIDIZED 2p orbital in an up-down direction (I label it 2py here) and ANOTHER going into-and-out-of-the-page (I label is 2pz here). ONE of the oxygens will make a pi bond with the 2py orbital and the OTHER oxygen will make a pi bond with the 2pz orbital. Sigma bonds are shown along the bond axis (end to end overlap)
Orbital Overlap Diagram of CO2 (carbon dioxide)
CO2 : ORBITAL OVERLAPPING DIAGRAM
Hybridisation of CO2
Structure of Carbon Dioxide (CO2)|| Hybridization Approach|| Chemistry Sciences
Hybridization of CO2 (Carbon in CO2, Oxygen in CO2)
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Orbital Overlap Diagram of Ethyne - Acetylene
Hybridization of CO2
Molecular Orbital Diagram of Polyatomic CO2 Molecules - Chemical Bonding & Molecular Structures
SCl6, ICl2-, ICl4+, CO2, C2H2 & C2H4 : ORBITAL OVERLAPPING DIAGRAM
This Is How We Do It.. CO2 hybridization with Elizabeth
Valence Bond Theory & Hybrid Atomic Orbitals
CO2 Hybridization: Hybrid Orbitals for CO2
Hybridization of CO2 (Carbon Dioxide)
Hybridisation of CO2|| #shorts
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Molecular orbitals of carbon dioxide part 2
Draw the Orbital Overlap Diagram for NH3 (ammonia)
Sp orbital Hybridization of Central Carbon in Carbon dioxide - Organic Chem #stemeducation #stem
Orbital Overlap Diagram for NH3 (Ammonia)
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
Hybridisation and Bonding in Carbon Dioxide
Комментарии
 0:06:54
0:06:54
 0:03:38
0:03:38
 0:04:55
0:04:55
 0:08:27
0:08:27
 0:02:19
0:02:19
 0:07:26
0:07:26
 0:10:55
0:10:55
 0:10:06
0:10:06
 0:06:20
0:06:20
 0:07:25
0:07:25
 0:07:09
0:07:09
 0:27:15
0:27:15
 0:06:11
0:06:11
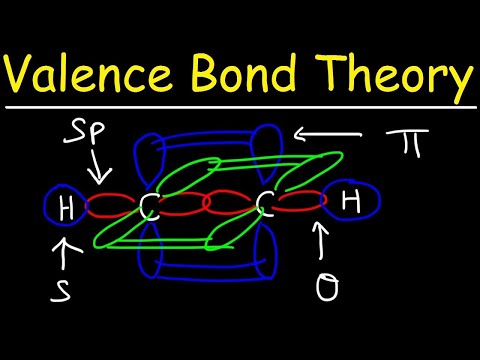 0:10:39
0:10:39
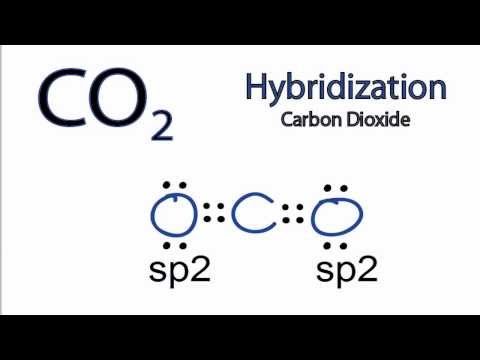 0:01:15
0:01:15
 0:01:45
0:01:45
 0:00:32
0:00:32
 0:13:48
0:13:48
 0:14:06
0:14:06
 0:07:07
0:07:07
 0:01:00
0:01:00
 0:06:13
0:06:13
 0:06:42
0:06:42
 0:12:08
0:12:08