filmov
tv
Orbital Overlap Diagram for N2

Показать описание
Here, we draw the orbitals that overlap to hold together a molecule of N2, nitrogen gas, molecular, nitrogen.
When two nitrogen atoms are bonded to each other, there is a SIGMA bond that is the result of an overlap between one sp hydridized orbital from each atom. There are, additionally two PI bonds, which results from a sideways overlap of leftover (unhybridized) 2p orbitals. There are lone pairs of electrons in the other sp hybrid orbitals.
When two nitrogen atoms are bonded to each other, there is a SIGMA bond that is the result of an overlap between one sp hydridized orbital from each atom. There are, additionally two PI bonds, which results from a sideways overlap of leftover (unhybridized) 2p orbitals. There are lone pairs of electrons in the other sp hybrid orbitals.
Orbital Overlap Diagram for N2
Hybridisation of N2
Hybridization of N2 (nitrogen)
Molecular Orbital (MO) Diagram for N2(-)
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Drawing Molecular Orbital Diagrams
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
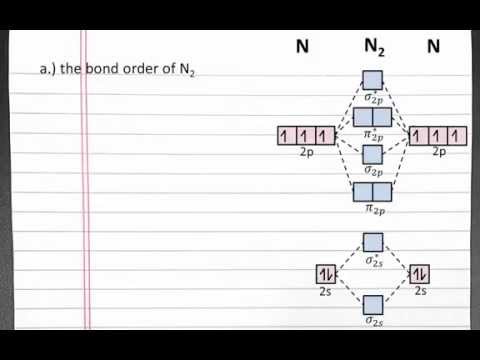
Molecular Orbital diagram of N2 molecule || MOT of NITROGEN MOLECULE
Molecular orbital diagram of N2 Molecules.
Molecular Orbital MO Diagram for N2(2-)
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
What is the hybridization of the nitrogen atoms in N2 | N2 Hybridization
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
9.5 Molecular Orbital Theory | General Chemistry
Molecular Orbital Diagram of B2, C2, and N2 Molecules.
N2 formation according to MOT | Molecular orbital Theory | 11th Class chemistry
How to Draw Molecular Orbital (MO) diagram of N2
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
Molecular Orbitals - Ions
Overlapping in H2, HF, F2, N2 and H2S molecules(VBT)
Formation of Nitrogen Molecule
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond strength, magnetic properties
Molecular Orbital Theory (MOT) , Quick Revision in 5 Minutes
Bonding in the N2 Molecule
Комментарии
 0:09:18
0:09:18
 0:04:00
0:04:00
 0:01:43
0:01:43
 0:04:11
0:04:11
 0:10:55
0:10:55
 0:11:05
0:11:05
 0:07:26
0:07:26
 0:05:50
0:05:50
 0:06:27
0:06:27
 0:04:30
0:04:30
 0:10:06
0:10:06
 0:01:16
0:01:16
 0:21:36
0:21:36
 0:45:53
0:45:53
 0:10:50
0:10:50
 0:18:26
0:18:26
 0:04:35
0:04:35
 0:06:42
0:06:42
 0:11:23
0:11:23
 0:04:26
0:04:26
 0:01:45
0:01:45
 0:05:51
0:05:51
 0:05:48
0:05:48
 0:09:01
0:09:01