filmov
tv
Orbital Overlap Diagram for NH3 (Ammonia)

Показать описание
The nitrogen atom at the centre of the molecule is sp3 hybridized. There are SIGMA bonds between each of three sp3 hybrid orbitals with hydrogen 1s orbitals. There is also a lone pair of electrons in the fourth sp3 hybrid orbital.
Orbital Overlap Diagram for NH3 (Ammonia)
Draw the Orbital Overlap Diagram for NH3 (ammonia)
lecture 10 part b MO diagram of NH3
nitrogen hybridization/ sp3 hybridization in ammonia, orbital overlap diagram of nh3.
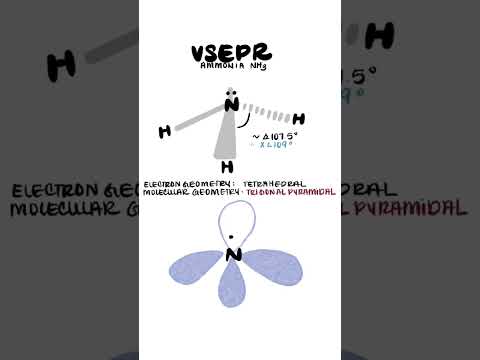
Ammonia Hybridization sp3
S2.2.16 - What is the hybridisation of nitrogen in ammonia?
Depicting Hybridization of Atomic Orbitals - Ammonia (NH3) 001
Ammonia NH3 Contour Diagram sp3 hybridization
NH3 Hybridization: Hybrid Orbitals for NH3 (ammonia)
Hybridization of NH3 (ammonia)
sp3 Orbital Hybridization of Nitrogen in Ammonia NH3 - Organic Chemistry #stemeducation #stem #chem
Orbital Overlap Diagram of Ethyne - Acetylene
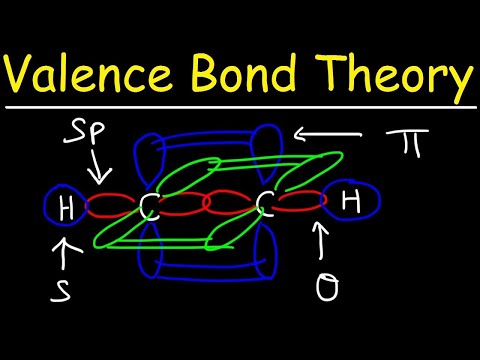
Valence Bond Theory & Hybrid Atomic Orbitals
MOT for Coordination compounds 6
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Construction of MO Diagrams for Simple Polyatomic Molecules
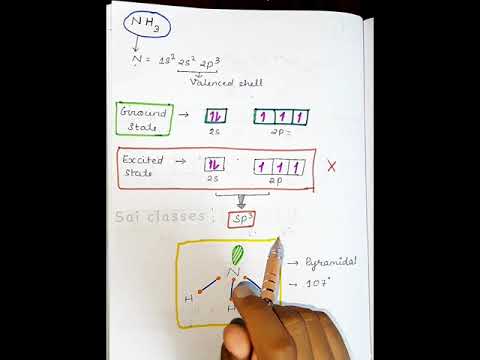
NH3 hybridization || basic chemistry class || #shorts #saiclasses
Drawing Molecular Orbital Diagrams
Hybridisation of NH3
| Super Concept | SP3 Hybridization In NH3 Compound | Shape | Orbital | 3D Model | Animation |
Overlap Diagrams
Video 7 Orbital Overlap Diagrams
Orbital overlap diagram of Ethanoic Acid (Acetic acid)
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Комментарии
 0:06:13
0:06:13
 0:07:07
0:07:07
 0:05:11
0:05:11
 0:10:46
0:10:46
 0:05:34
0:05:34
 0:04:35
0:04:35
 0:05:33
0:05:33
 0:05:00
0:05:00
 0:00:44
0:00:44
 0:01:57
0:01:57
 0:00:54
0:00:54
 0:06:20
0:06:20
 0:10:39
0:10:39
 0:13:10
0:13:10
 0:10:55
0:10:55
 0:37:51
0:37:51
 0:01:00
0:01:00
 0:11:05
0:11:05
 0:05:20
0:05:20
 0:00:59
0:00:59
 0:07:05
0:07:05
 0:20:55
0:20:55
 0:08:15
0:08:15
 0:10:06
0:10:06