filmov
tv
Steps in Drawing Orbital Overlapping & How to Draw Orbital Overlapping?

Показать описание
SDS SK015 Topic 4.3 (Part 4)
a) Prior Knowledge 00:35
b) Steps to Draw Orbital Overlapping 03:10
c) Example 1 beryllium fluoride 05:43
d) Example 2 boron trichloride 10:54
e) Example 3 methane 15:30
f) Example 4 phosphorus pentachloride 19:19
g) Example 5 sulphur hexafluoride 25:17
SDS SK015
Topic 4: Chemical Bonding
Subtopic 4.3: Orbital Overlapping and Hybridisation
Remember to drop your comments and feedback.
a) Prior Knowledge 00:35
b) Steps to Draw Orbital Overlapping 03:10
c) Example 1 beryllium fluoride 05:43
d) Example 2 boron trichloride 10:54
e) Example 3 methane 15:30
f) Example 4 phosphorus pentachloride 19:19
g) Example 5 sulphur hexafluoride 25:17
SDS SK015
Topic 4: Chemical Bonding
Subtopic 4.3: Orbital Overlapping and Hybridisation
Remember to drop your comments and feedback.
Steps in Drawing Orbital Overlapping & How to Draw Orbital Overlapping?
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Orbital Overlap Diagram of Ethyne - Acetylene
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
Understanding Overlapping Atomic Orbitals
Drawing Molecular Orbital Diagrams
Draw the Orbital Overlap Diagram for NH3 (ammonia)
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
EASY Method to Find the Hybridization of an Atom | Chemistry |
Molecule with Multiple Central Atoms - Draw Hybridisation Process & Orbital Overlapping
Orbitals: Crash Course Chemistry #25
Valence Bond Theory & Hybrid Atomic Orbitals
Central Atom with Pi Bond - Draw Hybridisation Process & Orbital Overlapping
How To Draw Lewis Structures
Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy |Crash Chemistry Academy...
Draw the Orbital Overlap Diagram of O2 (Oxygen gas)
Orbital overlap diagram of Ethanoic Acid (Acetic acid)
Orbital Overlap Diagram for N2
Central Atom with Lone Pair - Draw Hybridisation Process & Orbital Overlapping
Video 7 Orbital Overlap Diagrams
How to Draw Orbital Diagrams and Hund's Rule | Study Chemistry With Us
Комментарии
 0:29:59
0:29:59
 0:10:06
0:10:06
 0:06:20
0:06:20
 0:07:26
0:07:26
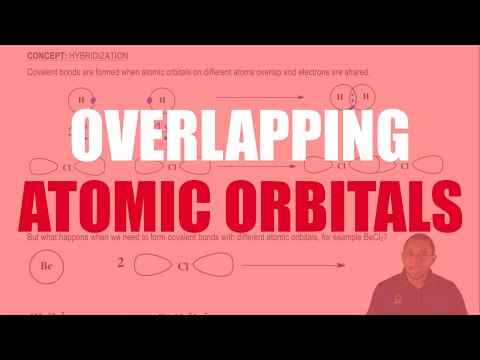 0:02:21
0:02:21
 0:11:05
0:11:05
 0:07:07
0:07:07
 0:06:17
0:06:17
 0:13:48
0:13:48
 0:07:54
0:07:54
 0:06:42
0:06:42
 0:04:08
0:04:08
 0:36:44
0:36:44
 0:10:52
0:10:52
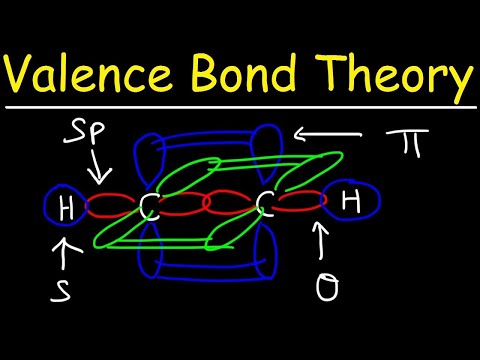 0:10:39
0:10:39
 0:47:01
0:47:01
 0:11:50
0:11:50
 0:14:28
0:14:28
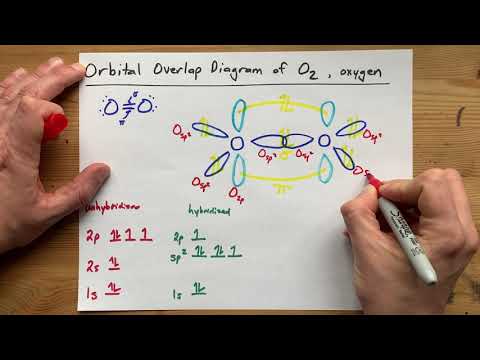 0:07:59
0:07:59
 0:08:15
0:08:15
 0:09:18
0:09:18
 0:48:01
0:48:01
 0:20:55
0:20:55
 0:05:37
0:05:37