filmov
tv
Orbital Overlap Diagram of CO (carbon monoxide)

Показать описание
Both the carbon AND the oxygen are "sp" hybridized, and therefore the two hybrid orbitals are arranged LINEARLY.
THEN, one of the leftover 2p orbitals is drawn in the "up-down" direction ... they 'overlap' to make one of the pi bonds (the "double" bond).
The other leftover 2p orbital is drawn in the "forward-backward" direction, and they overlap to make the second pi bond (the "triple" bond).
THEN, one of the leftover 2p orbitals is drawn in the "up-down" direction ... they 'overlap' to make one of the pi bonds (the "double" bond).
The other leftover 2p orbital is drawn in the "forward-backward" direction, and they overlap to make the second pi bond (the "triple" bond).
Orbital Overlap Diagram of CO (carbon monoxide)
Orbital Overlap Diagram of Ethyne - Acetylene
Orbital Overlap Diagram of CO2 (carbon dioxide)
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Drawing Molecular Orbital Diagrams
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
Hybridization of CO (Carbon Monoxide)
Valence Bond Theory & Hybrid Atomic Orbitals
Hybridization, Orbital Overlap, and Bond Length
Understanding Overlapping Atomic Orbitals
hybridization of CO (Carbon Monoxide) in english
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
Orbital overlap diagram of Ethanoic Acid (Acetic acid)
Molecular Orbital Diagram of Polyatomic CO2 Molecules - Chemical Bonding & Molecular Structures
Molecular Orbital Theory | Chemistry
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry
Hybridisation of CO2
Types of Bonding in Transition Metal Systems and Simple Ligands
Determining Orbital Overlap for Covalent Bonding
CO2 : ORBITAL OVERLAPPING DIAGRAM
Molecular Orbital Theory | mo diagram of NO | mo diagram of CO |mo diagram of CN | mo diagram of HF
Molecular Orbital Diagram for Carbon Monoxide
Комментарии
 0:06:27
0:06:27
 0:06:20
0:06:20
 0:06:54
0:06:54
 0:06:42
0:06:42
 0:07:54
0:07:54
 0:10:06
0:10:06
 0:11:05
0:11:05
 0:21:36
0:21:36
 0:01:31
0:01:31
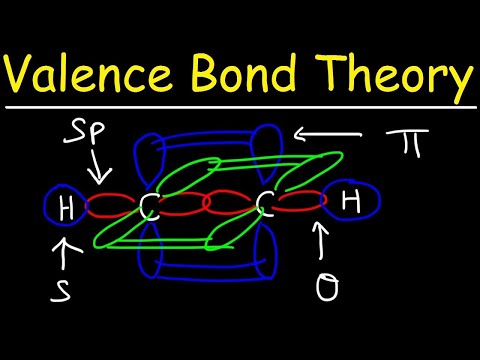 0:10:39
0:10:39
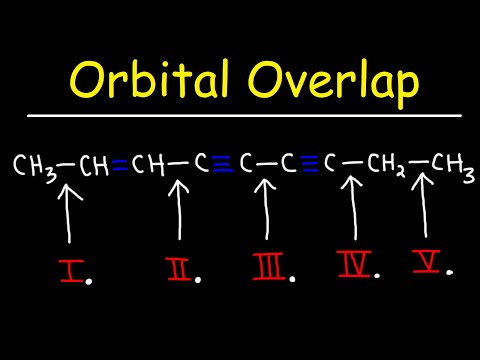 0:08:03
0:08:03
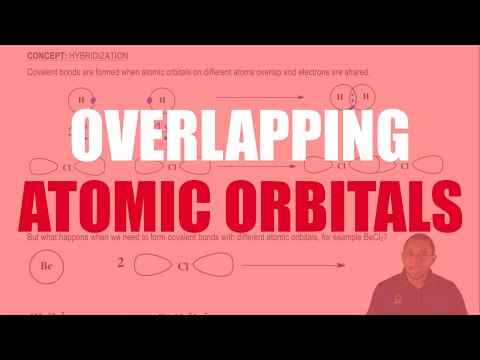 0:02:21
0:02:21
 0:04:55
0:04:55
 0:07:26
0:07:26
 0:08:15
0:08:15
 0:07:09
0:07:09
 0:19:13
0:19:13
 0:06:17
0:06:17
 0:04:55
0:04:55
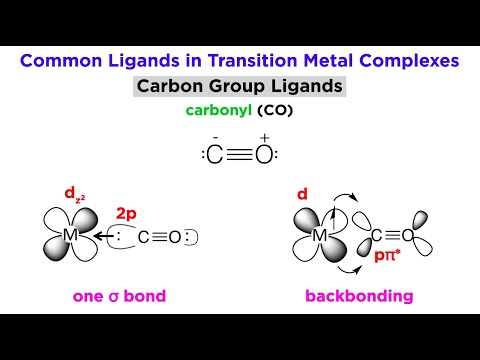 0:11:54
0:11:54
 0:05:55
0:05:55
 0:03:38
0:03:38
 0:12:19
0:12:19
 0:22:37
0:22:37