filmov
tv
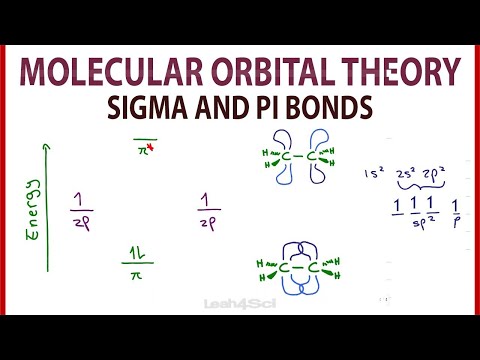
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)

Показать описание
Ethene, which is two carbon atoms double bonded and two hydrogen atoms on EACH carbon (four hydrogen atoms total), requires ONE pi bond. This means each carbon is sp2 hybridized. This in turn means you have three hybridized sp2 orbitals arranged in a trigonal-planar way around each carbon, AND you have a leftover p orbital on each as well. You can show the overlap of these orbitals to make sigma and pi bonds.
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond)
Orbital Overlap Diagram of Ethyne - Acetylene
orbital picture of ethylene
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
Hybridisation of C2H4 || sp2 hybridisation || Formation of Ethene molecule
C2H4 Hybridization (Ethylene)
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Q. Draw molecular orbital diagram for ethane, ethene & ethyne molecules?
Carbon hybridization,sp2 hybridization in carbon,ethylene, orbital overlap diagram of c2h4.
sp2 hybridisation, Structure of ethyleneC2H4
Orbital Overlap Diagram for CH2O (methanal, formaldehyde)
ETHYLENE STRUCTURE /BONDING/ with 3D Animation / CH2=CH2 molecule/ sp2 Hybridisation
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
SCl6, ICl2-, ICl4+, CO2, C2H2 & C2H4 : ORBITAL OVERLAPPING DIAGRAM
Carbon hybridization/sp hybridization in carbon, acetylene, orbital overlap diagram of c2h4.
Bonding in Ethene (Ethylene)
Hybridization, Hybrid Orbitals, and Bonding Diagrams
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
Double Covalent bond. Example: C2H4 Molecule
16.2a Introduction to Pi Molecular Orbitals Ethylene
SP2 Hybridization, Formation of Ethene (C2H4) Molecule | MYEH-077 | Chemical Bonding | CHEMISTRY.
hybridization in ethylene #ethylene #alkene #K2chemistryclass
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry
Комментарии
 0:07:26
0:07:26
 0:06:20
0:06:20
 0:03:03
0:03:03
 0:10:06
0:10:06
 0:05:41
0:05:41
 0:02:08
0:02:08
 0:10:55
0:10:55
 0:05:11
0:05:11
 0:09:21
0:09:21
 0:01:01
0:01:01
 0:06:42
0:06:42
 0:03:25
0:03:25
 0:07:54
0:07:54
 0:13:48
0:13:48
 0:27:15
0:27:15
 0:08:40
0:08:40
 0:06:24
0:06:24
 0:14:14
0:14:14
 0:13:19
0:13:19
 0:00:37
0:00:37
 0:06:57
0:06:57
 0:00:51
0:00:51
 0:00:59
0:00:59
 0:08:22
0:08:22