filmov
tv
What Are Catalysts? | Reactions | Chemistry | FuseSchool

Показать описание
What Are Catalysts? | Reactions | Chemistry | FuseSchool
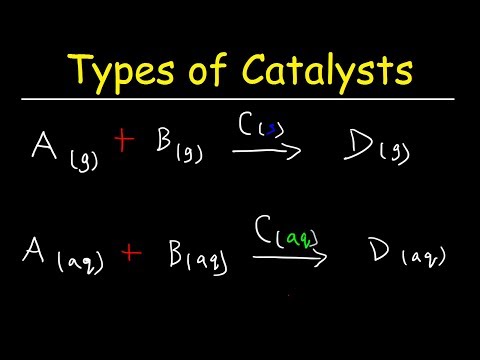
Learn the basics about Catalysts. What are catalysts? How do catalysts work? How are catalysts used in industry? Find out more in this video!
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Learn the basics about Catalysts. What are catalysts? How do catalysts work? How are catalysts used in industry? Find out more in this video!
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
What Are Catalysts? | Reactions | Chemistry | FuseSchool
Chemistry - 3Sec - The effect of catalysts on the rate of chemical reactions
Energy Diagrams, Catalysts, and Reaction Mechanisms
Catalysts in Chemistry Explained
What are Catalysts | Catalyst kya hota hai ? | Chemistry Catalyst
Homogeneous vs Heterogeneous Catalysts - Basic Introduction
Introduction to Catalysis and Catalysts // Reactor Engineering - Class 135
What Are Catalysts - Reactions | Concept in English | Chemistry
Silver Based Catalysts - A Game Changer for Green Hydrogen
What is a catalyst and how does catalysis work?
Catalysts and Homogeneous and Heterogeneous Catalysis (A-Level IB Chemistry)
How does an exhaust catalytic converter work?
What are Catalysts?
How To Identify The Intermediate & Catalyst In a Reaction Mechanism - Kinetics Chemistry
What is the role of catalysts in chemical reactions?
How Catalysts Affect Rate Of Reaction - GCSE Chemistry | kayscience.com
What are Catalysts
What Are Catalysts? | Reactions | Chemistry | Learners Club by time to change
Catalysts and Activation Energy
Chemical Reaction Rates and Catalysts
Key Stage 3 Chemistry - Catalysts
Catalysts | Reaction rates and equilibrium | High school chemistry | Khan Academy
What Are Catalysts? | Reactions | Chemistry | DICE-21
⏳ Science Concept in 2 mins ⏳| What are Catalysts❓❓ Explained by Prashant Bhaiya 😎
Комментарии
 0:03:34
0:03:34
 0:01:55
0:01:55
 0:05:23
0:05:23
 0:04:21
0:04:21
 0:02:40
0:02:40
 0:01:34
0:01:34
 0:04:47
0:04:47
 0:02:52
0:02:52
 0:01:42
0:01:42
 0:03:55
0:03:55
 0:10:57
0:10:57
 0:01:48
0:01:48
 0:07:31
0:07:31
 0:05:15
0:05:15
 0:00:35
0:00:35
 0:06:51
0:06:51
 0:05:11
0:05:11
 0:03:09
0:03:09
 0:03:44
0:03:44
 0:09:00
0:09:00
 0:04:19
0:04:19
 0:05:03
0:05:03
 0:27:01
0:27:01
 0:02:11
0:02:11