filmov
tv
Catalysts and Homogeneous and Heterogeneous Catalysis (A-Level IB Chemistry)

Показать описание
Outlining the role of catalysts in the rates of reactions, linked to activation energy and Maxwell-Boltzmann distribution curves. Homogeneous and Heterogeneous catalysts and catalysis is covered, with examples of each given including the reaction of iodide ions with thiosulfate ions (Fe2+ catalyst) and the contact process between sulfur dioxide and oxygen (V2O5 catalyst).
For AQA, OCR, Edexcel and CIE
Recap: 00:26
Catalysts: 01:33
Activation Energy: 03:01
How catalysts work: 04:29
Homogeneous Catalysts: 06:37
EXAMPLE - Homogeneous Catalyst: 06:05
Heterogeneous Catalysts: 08:09
EXAMPLE - Heterogeneous Catalyst: 09:05
Summary: 09:26
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
For AQA, OCR, Edexcel and CIE
Recap: 00:26
Catalysts: 01:33
Activation Energy: 03:01
How catalysts work: 04:29
Homogeneous Catalysts: 06:37
EXAMPLE - Homogeneous Catalyst: 06:05
Heterogeneous Catalysts: 08:09
EXAMPLE - Heterogeneous Catalyst: 09:05
Summary: 09:26
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
Homogeneous vs Heterogeneous Catalysts - Basic Introduction
Catalysts and Homogeneous and Heterogeneous Catalysis (A-Level IB Chemistry)
What is a Homogeneous Catalyst?
Homogeneous and Heterogeneous Mixture | Chemistry
Difference Between Homogeneous Catalysis and Heterogeneous Catalysis - Surface Chemistry
homogeneous and heterogeneous catalysts
homogeneous and heterogeneous mixture 🧬 school science experiment
Catalysis - Homogeneous vs. Heterogeneous
Chemical Kinetics | CUET PG PYQ Series | Physical Chemistry | Sonia Mam Chemistry
Homogeneous and Heterogeneous catalysis
Principles of Homogeneous Catalysis
Catalysts: Homogeneous & Heterogeneous | A-level Chemistry | OCR, AQA, Edexcel
How Does Homogeneous and Heterogeneous Catalysts Work?
Chemical Kinetics Made Easy: Homogeneous and Heterogeneous Catalysts Episode #13
HETEROGENEOUS AND HOMOGENEOUS CATALYST OUTCOME REACTION RATES
Alcohol se experiment I Homogeneous and heterogeneous mixture #shorts #experiment #science
Simple science - Video #4 - Catalysts: Heterogeneous and homogeneous
10 Examples of Homogeneous Mixtures and Heterogeneous Mixtures
homogeneous and heterogeneous catalysis
Types of catalysts | Kinetics | AP Chemistry | Khan Academy
12.77 | What is the difference between a homogeneous and a heterogeneous catalyst?
Homogeneous and heterogeneous. Science concept explained #homogenous #science #ncertscience #learn
Concept for homogeneous and heterogeneous catalysis | Class 12 chemistry #shorts #viral
WHAT ARE THE SIMILARITIES AND DIFFERENCES BETWEEN HOMOGENEOUS AND HETEROGENEOUS CATALYSTS?
Комментарии
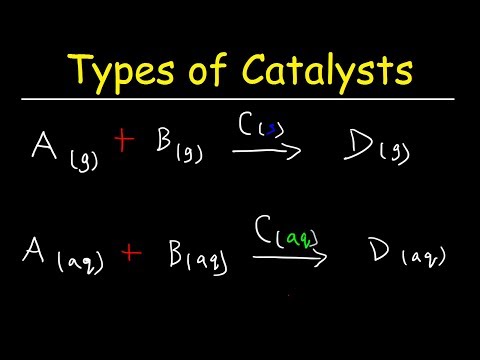 0:01:34
0:01:34
 0:10:57
0:10:57
 0:01:36
0:01:36
 0:05:01
0:05:01
 0:11:00
0:11:00
 0:05:09
0:05:09
 0:00:31
0:00:31
 0:01:02
0:01:02
 0:24:14
0:24:14
 0:04:59
0:04:59
 0:08:25
0:08:25
 0:07:57
0:07:57
 0:03:19
0:03:19
 0:05:40
0:05:40
 0:01:22
0:01:22
 0:01:00
0:01:00
 0:04:26
0:04:26
 0:03:41
0:03:41
 0:14:47
0:14:47
 0:04:59
0:04:59
 0:09:30
0:09:30
 0:01:00
0:01:00
 0:00:12
0:00:12
 0:03:57
0:03:57