filmov
tv
What Are Catalysts - Reactions | Concept in English | Chemistry

Показать описание
Catalysis is the process of change in rate of a chemical reaction by adding a substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
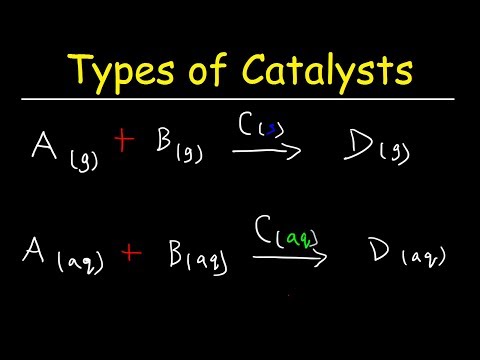
Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category.
Credits: Fuseschool
#chemistry #chemical #catalyst #catalysis #chemicalbondingandmolecularstructure #chemicalbonding #chemicalbond #basic #basicchemistry #chemistryclasses #chemistryshortcuts #chemistrynotes #chemistry9th #chemistrystudent #chemistrystudents #school #learning #learn #learnchemistry #youtube #upload #learnchemistryeasy #schools #learneasy
Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category.
Credits: Fuseschool
#chemistry #chemical #catalyst #catalysis #chemicalbondingandmolecularstructure #chemicalbonding #chemicalbond #basic #basicchemistry #chemistryclasses #chemistryshortcuts #chemistrynotes #chemistry9th #chemistrystudent #chemistrystudents #school #learning #learn #learnchemistry #youtube #upload #learnchemistryeasy #schools #learneasy
 0:03:34
0:03:34
 0:01:55
0:01:55
 0:04:21
0:04:21
 0:05:23
0:05:23
 0:01:34
0:01:34
 0:05:15
0:05:15
 0:05:11
0:05:11
 0:07:31
0:07:31
 1:59:42
1:59:42
 0:04:38
0:04:38
 0:02:15
0:02:15
 0:03:55
0:03:55
 0:05:03
0:05:03
 0:05:21
0:05:21
 0:02:52
0:02:52
 0:04:19
0:04:19
 0:04:10
0:04:10
 0:09:00
0:09:00
 0:04:47
0:04:47
 0:03:14
0:03:14
 0:02:40
0:02:40
 0:03:34
0:03:34
 0:10:57
0:10:57
 0:02:32
0:02:32