filmov
tv
Catalysts | Reaction rates and equilibrium | High school chemistry | Khan Academy

Показать описание
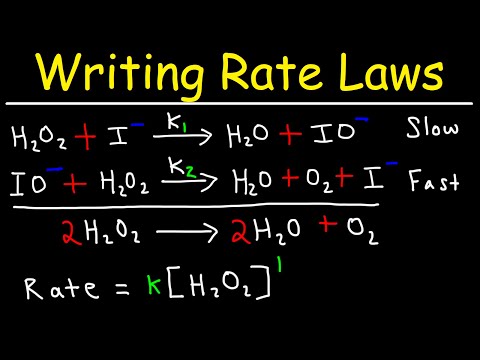
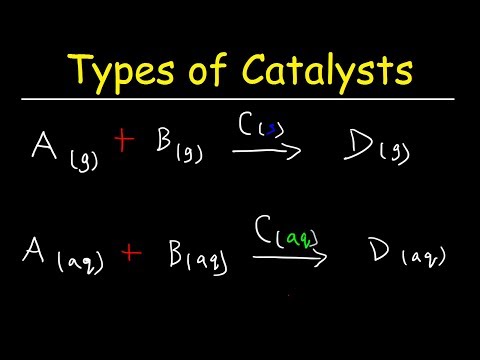
Catalysts are chemicals that help chemical reactions proceed faster rate. But the catalyst itself is not consumed in the reaction. There are a variety of ways that a catalyst causes the rate to increase. In all cases, the catalyst speeds up a reaction by lowering the activation energy. An enzyme is a substance produced by a living organism that acts a catalyst in biochemical reactions.
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
 0:03:34
0:03:34
 0:05:23
0:05:23
 0:03:37
0:03:37
 0:01:55
0:01:55
 0:05:03
0:05:03
 0:05:15
0:05:15
 0:02:36
0:02:36
 0:04:56
0:04:56
 0:19:00
0:19:00
 0:05:15
0:05:15
 0:06:14
0:06:14
 0:09:00
0:09:00
 0:09:08
0:09:08
 0:02:15
0:02:15
 0:04:45
0:04:45
 0:01:38
0:01:38
 0:18:48
0:18:48
 0:18:52
0:18:52
 0:00:56
0:00:56
 0:08:03
0:08:03
 0:03:45
0:03:45
 0:08:11
0:08:11
 0:01:34
0:01:34
 0:03:07
0:03:07