filmov
tv
Homogeneous vs Heterogeneous Catalysts - Basic Introduction
Показать описание
This video provides a basic introduction into homogeneous and heterogeneous catalysts. A Homogeneous catalyst exists in the same phase as the reactants and products. A heterogeneous catalyst exists in a different phase as the reactants and products.
Chem Kinetics Formula Sheet:
_____________________________
Chemical Kinetics - Initial Rate Method:
Rate Constant k - Find The Units:
Integrated Rate Laws - 1st & 2nd Order:
Reaction Rate Factors:
Collision Theory & Activation Energy:
___________________________________
Potential Energy Diagrams:
Elementary Rate Laws:
Rate Laws of Reaction Mechanisms:
Intermediates & Catalysts:
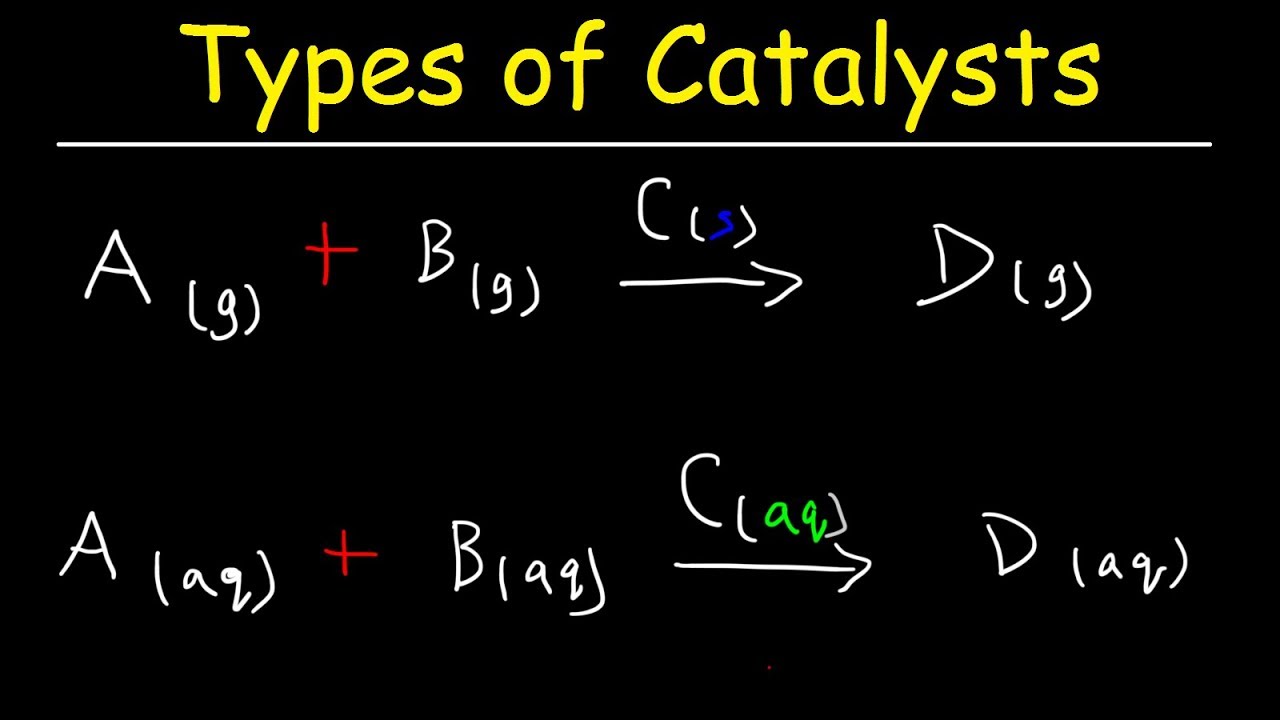
Types of Catalysts:
____________________________________
The Equilibrium Expression:
Calculating Kp From Kc:
Chemical Equilibrium & Ice Tables:
Le Chatelier's Principle:
Acids and Bases - Introduction:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Chem Kinetics Formula Sheet:
_____________________________
Chemical Kinetics - Initial Rate Method:
Rate Constant k - Find The Units:
Integrated Rate Laws - 1st & 2nd Order:
Reaction Rate Factors:
Collision Theory & Activation Energy:
___________________________________
Potential Energy Diagrams:
Elementary Rate Laws:
Rate Laws of Reaction Mechanisms:
Intermediates & Catalysts:
Types of Catalysts:
____________________________________
The Equilibrium Expression:
Calculating Kp From Kc:
Chemical Equilibrium & Ice Tables:
Le Chatelier's Principle:
Acids and Bases - Introduction:
______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:01:34
0:01:34
 0:10:57
0:10:57
 0:01:22
0:01:22
 0:01:36
0:01:36
 0:07:57
0:07:57
 0:05:01
0:05:01
 0:24:03
0:24:03
 0:03:22
0:03:22
 0:01:02
0:01:02
 0:08:48
0:08:48
 0:11:00
0:11:00
 0:05:09
0:05:09
 0:14:54
0:14:54
 0:00:31
0:00:31
 0:04:59
0:04:59
 0:14:47
0:14:47
 0:04:40
0:04:40
 0:09:30
0:09:30
 0:04:59
0:04:59
 0:03:41
0:03:41
 0:07:50
0:07:50
 0:09:14
0:09:14
 0:05:40
0:05:40
 0:08:23
0:08:23