filmov
tv
Hydrogen Bonding (A-Level Chemistry)

Показать описание
Outlining hydrogen bonds as intermolecular forces, how they arise and factors that influence their strength. The boiling points of ammonia and phosphine are compared, the boiling points of water and hydrogen disulfide are compared and the melting points of hydrogen fluoride and hydrogen chloride are compared.
Recap: 00:23
Hydrogen Bonds: 02:16
Water and Hydrogen Disulfide: 04:47
Ammonia and Phosphine: 05:29
Hydrogen Fluoride and Hydrogen Chloride: 05:55
Summary: 06:25
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
Recap: 00:23
Hydrogen Bonds: 02:16
Water and Hydrogen Disulfide: 04:47
Ammonia and Phosphine: 05:29
Hydrogen Fluoride and Hydrogen Chloride: 05:55
Summary: 06:25
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
Hydrogen Bonding (A-Level Chemistry)
Hydrogen Bonds In Water Explained - Intermolecular Forces
Hydrogen Bonding - Revision for A-Level Chemistry
A Level Chemistry Hydrogen Bonding
Hydrogen bonding | Intermolecular forces and properties | AP Chemistry | Khan Academy
Hydrogen Bonding - AS Chemistry
Intermolecular Forces Explained | A level Chemistry
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubilit...
What are the Four Levels of Protein Structure?
Hydrogen Bonding | Chemistry
Identifying Hydrogen Bond Donors & Acceptors
Hydrogen Bonds - What Are Hydrogen Bonds - How Do Hydrogen Bonds Form
AQA A-Level Chemistry - Intermolecular Forces
Intermolecular Forces and Boiling Points
How to Identify the Intermolecular Force a Compound Has: London Dispersion, Dipole Dipole, H-Bonding
Properties of Water: Hydrogen Bonding | A-level Biology | OCR, AQA, Edexcel
Sigma and Pi Bonds (A-Level Chemistry)
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Hydrogen bonding | Explanation of hydrogen bonding with examples | ch#4 | 11th class Chemistry
Intermolecular Forces
Intermolecular Forces | A-level Chemistry | OCR, AQA, Edexcel
What Are Intermolecular Forces | Properties of Matter | Chemistry | FuseSchool
Ionic and Covalent Bonds, Hydrogen Bonds, van der Waals - 4 types of Chemical Bonds in Biology
Electronegativity & Polarity | Explained | A level Chemistry
Комментарии
 0:07:47
0:07:47
 0:10:54
0:10:54
 0:03:45
0:03:45
 0:07:19
0:07:19
 0:06:39
0:06:39
 0:10:40
0:10:40
 0:22:03
0:22:03
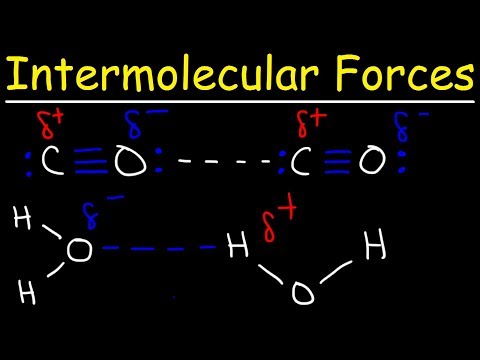 0:10:40
0:10:40
 0:14:51
0:14:51
 0:06:31
0:06:31
 0:05:36
0:05:36
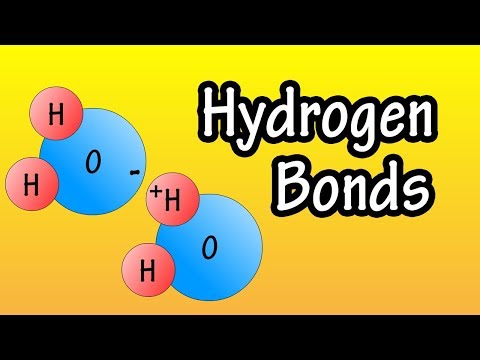 0:02:48
0:02:48
 0:37:01
0:37:01
 0:10:54
0:10:54
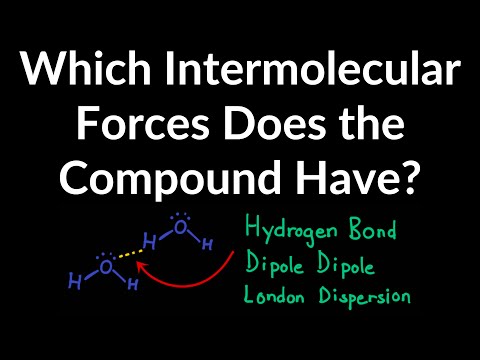 0:05:37
0:05:37
 0:10:37
0:10:37
 0:08:10
0:08:10
 0:03:33
0:03:33
 0:19:23
0:19:23
 0:14:17
0:14:17
 0:21:25
0:21:25
 0:05:19
0:05:19
 0:08:50
0:08:50
 0:11:53
0:11:53