filmov
tv
Electronegativity & Polarity | Explained | A level Chemistry

Показать описание
Electronegativity & Polarity Explained
A level Chemistry.
Shapes of Molecules Explained:
Intermolecular forces Question Walkthrough:
Bonding Question Walkthrough:
Bonding Multiple Choice Question Walkthrough:
Intermolecular Forces Explained:
00:26 Covalent Bonds
01:26 Sigma and Pi bonds
02:38 Unequal Electron Sharing
03:37 Electronegativity
05:13 Polar Bonds
08:15 Polar Molecules
09:30 Polar Bonds...Non Polar Molecules
A level Chemistry.
Shapes of Molecules Explained:
Intermolecular forces Question Walkthrough:
Bonding Question Walkthrough:
Bonding Multiple Choice Question Walkthrough:
Intermolecular Forces Explained:
00:26 Covalent Bonds
01:26 Sigma and Pi bonds
02:38 Unequal Electron Sharing
03:37 Electronegativity
05:13 Polar Bonds
08:15 Polar Molecules
09:30 Polar Bonds...Non Polar Molecules
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Electronegativity & Polarity | Explained | A level Chemistry
Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice Problems
Electronegativity
Electronegativity, Basic Introduction, Periodic Trends - Which Element Is More Electronegative?
Polar & Non-Polar Molecules: Crash Course Chemistry #23
Electronegativity & Bond Polarity | A-level Chemistry | OCR, AQA, Edexcel
Electronegativity & Polarity Explained
Polar & Non-Polar Covalent Bonds | Easy Explanation with Examples|9th Chemistry
Electronegativity Grade 11 Chemistry Polarity of Bonds
Electronegativity and Bond Polarity
Electronegativity: Polar Bonds
What is Electronegativity?? | Electronegativity Explained!!! | Polar Bond |Organic Chemistry
Polar And Nonpolar Covalent Bonds: Easy Explanation With Examples - Chemistry Basics
Electronegativity and Polarity (Part 1) | A-Level Chemistry
Polar Covalent Bonds and Electronegativity
3 main factors affecting polarity #shorts #ochem
Electronegativity and Bond Polarity, PhET Simulation - Revision for A-Level Chemistry
Electronegativity and Bond Polarity - Chemistry Tutorial
Electronegativity | Periodic Trends | Chemistry
9.6 Electronegativity & Bond Polarity
Electronegativity and Polarity
Polar And NonPolar Molecules | Chemistry
Electronegativity, Dipole Moment, Polar Covalent Bond Explained
Комментарии
 0:03:33
0:03:33
 0:11:53
0:11:53
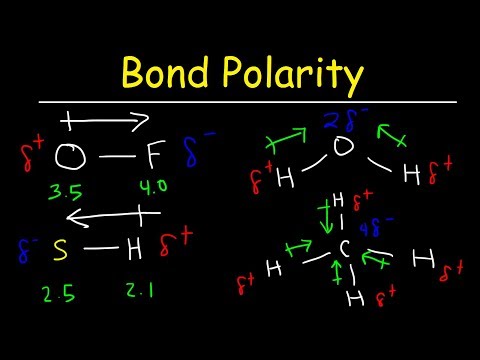 0:11:21
0:11:21
 0:02:12
0:02:12
 0:11:42
0:11:42
 0:10:46
0:10:46
 0:15:45
0:15:45
 0:03:33
0:03:33
 0:11:57
0:11:57
 0:16:15
0:16:15
 0:01:45
0:01:45
 0:07:29
0:07:29
 0:07:09
0:07:09
 0:04:52
0:04:52
 0:09:55
0:09:55
 0:13:13
0:13:13
 0:01:01
0:01:01
 0:11:25
0:11:25
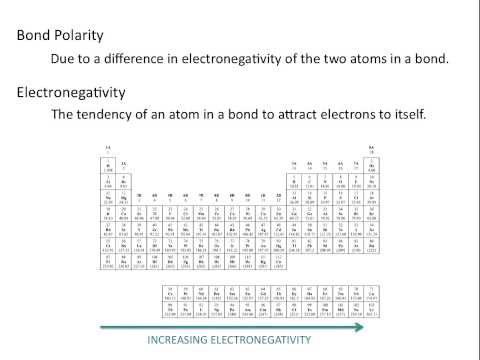 0:07:37
0:07:37
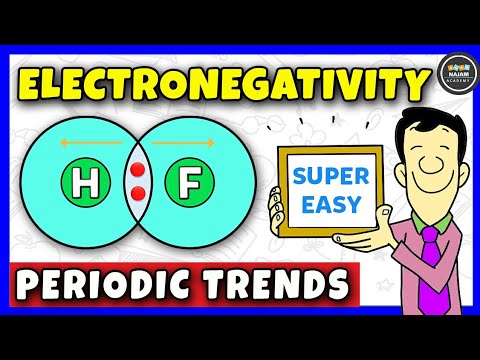 0:10:00
0:10:00
 0:18:32
0:18:32
 0:02:42
0:02:42
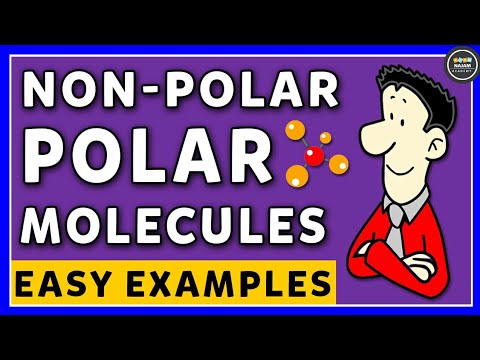 0:08:47
0:08:47
 0:03:17
0:03:17