filmov
tv
Identifying Hydrogen Bond Donors & Acceptors

Показать описание
Practice identifying organic molecules as hydrogen bond donors and/or acceptors.
Identifying Hydrogen Bond Donors & Acceptors
How to identify Hydrogen bond Donors and Acceptors - worked examples
Identifying Hydrogen Bond Donors
Topic 2.8 - H bond donors and acceptors
CHEM 405 - Hydrogen Bond Acceptors and Donors
Organic Chemistry Functional Groups - Polarity and Hydrogen Bond donators and acceptors
How to Tell Which Molecules Hydrogen Bond
Identify the hydrogen-bond donors and acceptors in each of the four bases on page 4
Identifying Hydrogen Bond Acceptors Part I
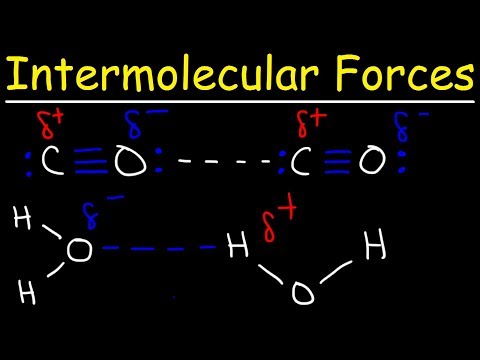
Hydrogen Bonds In Water Explained - Intermolecular Forces
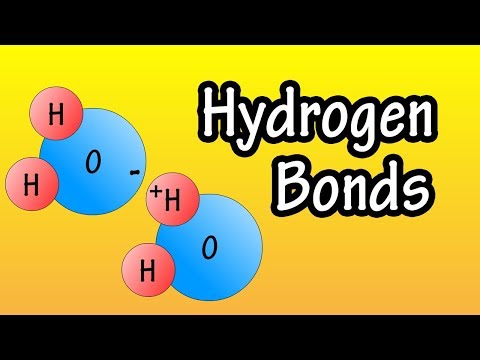
Hydrogen Bonds - What Are Hydrogen Bonds - How Do Hydrogen Bonds Form
Lecture 02, concept 16: Hydrogen bonds - orbitals, donors and acceptor
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubilit...
Hydrogen Bonding
Hydrogen Bonding
Chapter 2 - pt1: Hydrogen Bonding
Hydrogen bonding | Intermolecular forces and properties | AP Chemistry | Khan Academy
Hydrogen Bonding | Chemistry
Identifying Hydrogen Bond Acceptors Part III
Problem 12.12 - Hydrogen Bonding
Intermolecular Forces - Hydrogen Bonding 002
Organic Chem Review: Identifying Hydrogen Bonding in Alcohols | Kaplan MCAT Prep
02.02 Hydrogen Bonding in Alcohols
Hydrogen Bonding F O N
Комментарии
 0:05:36
0:05:36
 0:03:45
0:03:45
 0:02:05
0:02:05
 0:07:49
0:07:49
 0:18:31
0:18:31
 0:03:03
0:03:03
 0:02:00
0:02:00
 0:00:33
0:00:33
 0:03:44
0:03:44
 0:10:54
0:10:54
 0:02:48
0:02:48
 0:04:58
0:04:58
 0:10:40
0:10:40
 0:03:10
0:03:10
 0:06:36
0:06:36
 0:13:00
0:13:00
 0:06:39
0:06:39
 0:06:31
0:06:31
 0:05:32
0:05:32
 0:18:42
0:18:42
 0:10:09
0:10:09
 0:08:59
0:08:59
 0:03:24
0:03:24
 0:03:55
0:03:55