filmov
tv
Ionic and Covalent Bonds, Hydrogen Bonds, van der Waals - 4 types of Chemical Bonds in Biology

Показать описание
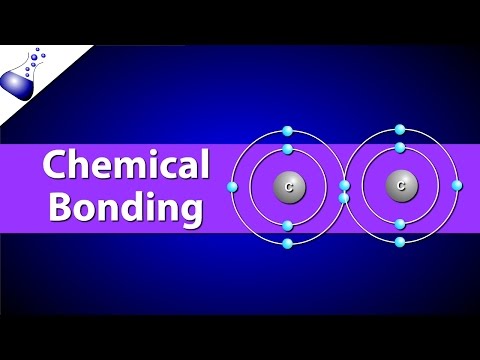
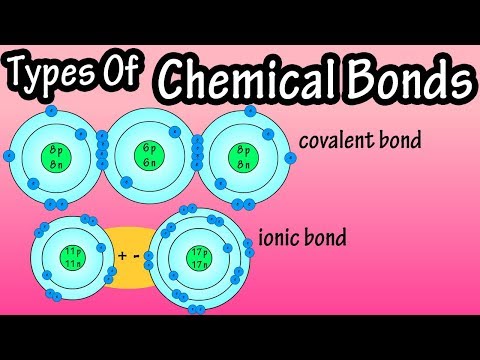
There are four types of chemical bonds essential for life to exist: Ionic Bonds, Covalent Bonds, Hydrogen Bonds, and van der Waals interactions. We need all of these different kinds of bonds to play various roles in biochemical interactions.
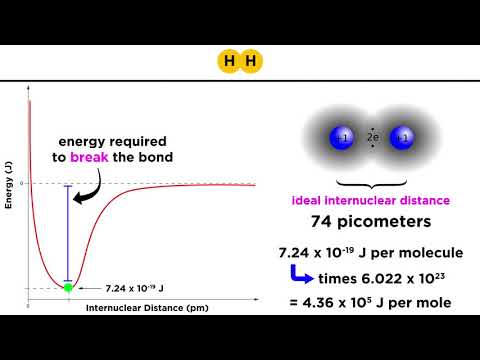
These bonds vary in their strengths. In Chemistry, we think of Ionic Bonds and Covalent bonds as having an overlapping range of strengths. But remember, in biochemistry, everything is happening in the context of water. This means Ionic bonds tend to dissociate in water. Thus, we will think of these bonds in the following order (strongest to weakest): Covalent, Ionic, Hydrogen, and van der Waals. Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces.”
Related Chemistry video:
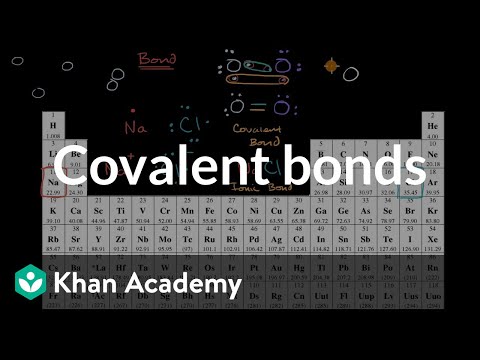
Ionic Bonds vs Covalent Bonds
SUBSCRIBE if you want to be notified of our next video!
Our series on Biology is aimed at the first-year college level, including pre-med students. These videos should also be helpful for students in challenging high school biology courses. Perfect for preparing for the AP Biology exam or the Biology SAT. Also appropriate for advanced homeschoolers. You can also follow along if you are just curious, and would like to know more about this fascinating subject.
♦♦♦♦♦♦♦♦♦♦
Our current biology textbook recommendation is Campbell Biology from Pearson.
Shop Amazon Used Textbooks - Save up to 90%
For lighter reading, we recommend:
I Contain Multitudes: The Microbes Within Us and a Grander View of Life by Ed Yong
Lab Girl by Hope Jahren
♦♦♦♦♦♦♦♦♦♦
This video was made possible by the generous donations of our Patrons on Patreon. We dedicate this video to our VIP Patron, Vishal Shah. We’re so thankful for your support!
Ways to support our channel:
► We also accept Bitcoin @ 1EttYyGwJmpy9bLY2UcmEqMJuBfaZ1HdG9
Thank you!
♦♦♦♦♦♦♦♦♦♦
Connect with us!
♦♦♦♦♦♦♦♦♦♦
Directed by Michael Harrison
Written and Produced by Kimberly Hatch Harrison
About our instructor:
Kimberly Hatch Harrison received degrees in Biology and English Literature from Caltech before working in pharmaceuticals research, developing drugs for autoimmune disorders. She then continued her studies in Molecular Biology (focusing on Immunology and Neurobiology) at Princeton University, where she began teaching as a graduate student. Her success in teaching convinced her to leave the glamorous world of biology research and turn to teaching full-time, accepting a position at an exclusive prep school, where she taught biology and chemistry for eight years. She is now the head writer and producer of Socratica Studios.
♦♦♦♦♦♦♦♦♦♦
Creative Commons Picture Credits:
Salt crystals
Author: W.J. Pilsak
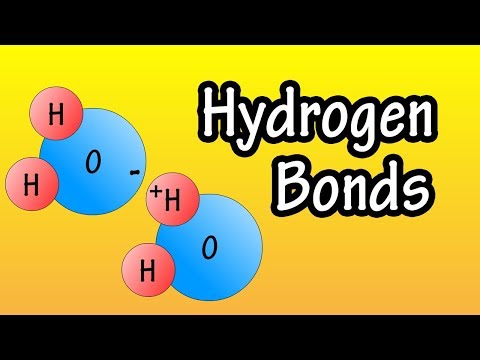
Hydrogen Bonding in water
Author: Qwerter
Products in this video:
These bonds vary in their strengths. In Chemistry, we think of Ionic Bonds and Covalent bonds as having an overlapping range of strengths. But remember, in biochemistry, everything is happening in the context of water. This means Ionic bonds tend to dissociate in water. Thus, we will think of these bonds in the following order (strongest to weakest): Covalent, Ionic, Hydrogen, and van der Waals. Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces.”
Related Chemistry video:
Ionic Bonds vs Covalent Bonds
SUBSCRIBE if you want to be notified of our next video!
Our series on Biology is aimed at the first-year college level, including pre-med students. These videos should also be helpful for students in challenging high school biology courses. Perfect for preparing for the AP Biology exam or the Biology SAT. Also appropriate for advanced homeschoolers. You can also follow along if you are just curious, and would like to know more about this fascinating subject.
♦♦♦♦♦♦♦♦♦♦
Our current biology textbook recommendation is Campbell Biology from Pearson.
Shop Amazon Used Textbooks - Save up to 90%
For lighter reading, we recommend:
I Contain Multitudes: The Microbes Within Us and a Grander View of Life by Ed Yong
Lab Girl by Hope Jahren
♦♦♦♦♦♦♦♦♦♦
This video was made possible by the generous donations of our Patrons on Patreon. We dedicate this video to our VIP Patron, Vishal Shah. We’re so thankful for your support!
Ways to support our channel:
► We also accept Bitcoin @ 1EttYyGwJmpy9bLY2UcmEqMJuBfaZ1HdG9
Thank you!
♦♦♦♦♦♦♦♦♦♦
Connect with us!
♦♦♦♦♦♦♦♦♦♦
Directed by Michael Harrison
Written and Produced by Kimberly Hatch Harrison
About our instructor:
Kimberly Hatch Harrison received degrees in Biology and English Literature from Caltech before working in pharmaceuticals research, developing drugs for autoimmune disorders. She then continued her studies in Molecular Biology (focusing on Immunology and Neurobiology) at Princeton University, where she began teaching as a graduate student. Her success in teaching convinced her to leave the glamorous world of biology research and turn to teaching full-time, accepting a position at an exclusive prep school, where she taught biology and chemistry for eight years. She is now the head writer and producer of Socratica Studios.
♦♦♦♦♦♦♦♦♦♦
Creative Commons Picture Credits:
Salt crystals
Author: W.J. Pilsak
Hydrogen Bonding in water
Author: Qwerter
Products in this video:
Комментарии
 0:08:50
0:08:50
 0:09:16
0:09:16
 0:03:33
0:03:33
 0:09:46
0:09:46
 0:02:15
0:02:15
 0:02:48
0:02:48
 0:11:00
0:11:00
 0:04:30
0:04:30
 0:12:37
0:12:37
 0:04:18
0:04:18
 0:03:34
0:03:34
 0:21:57
0:21:57
 0:03:00
0:03:00
 0:02:37
0:02:37
 0:05:33
0:05:33
 0:03:25
0:03:25
 0:05:43
0:05:43
 0:07:04
0:07:04
 0:02:58
0:02:58
 0:03:21
0:03:21
 0:05:36
0:05:36
 0:05:47
0:05:47
 0:03:03
0:03:03
 0:06:51
0:06:51