filmov
tv
What Are Intermolecular Forces | Properties of Matter | Chemistry | FuseSchool

Показать описание
What Are Intermolecular Forces | Properties of Matter | Chemistry | FuseSchool
Learn what intermolecular forces are, the three most common types and the differences between them.
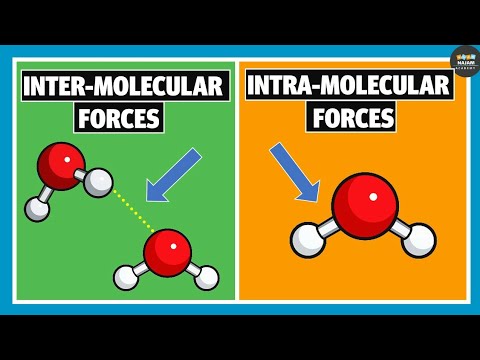
An intermolecular force is simply an attractive force between neighbouring molecules.
There are three common types of intermolecular force: permanent dipole-dipole forces, hydrogen bonds and van der Waals' forces. These three forces are much weaker than ionic or covalent bonds which bind atoms and ions together in elements and compounds.
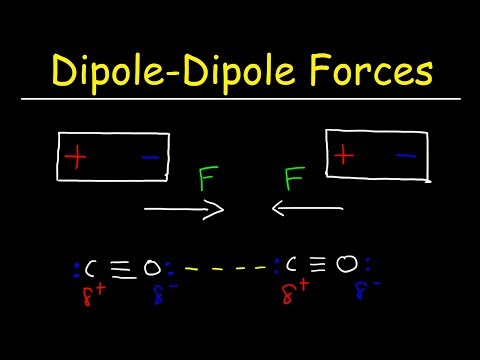
Permanent dipole-dipole forces: A polar molecule is one in which there is a permanent dipole, arising usually because the different atoms in the molecule have different electro-negativities.
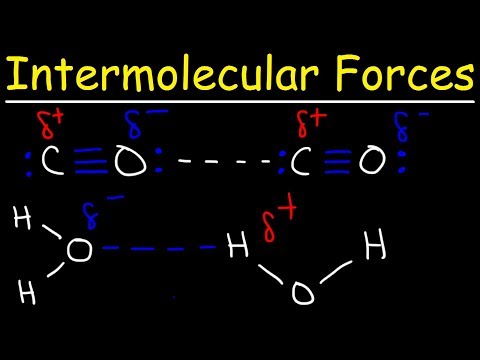
Hydrogen chloride is a polar molecule as the pair of electrons in the H---Cl bond are nearer the Cl atom because it has a greater electronegativity than the H atom. The two electrons of the covalent bond between the hydrogen and chlorine atoms are nearer the chlorine atom because of its greater electronegativity. Thus there will be an attraction between the chlorine atom of one molecule and the hydrogen atom of a neighbouring molecule.
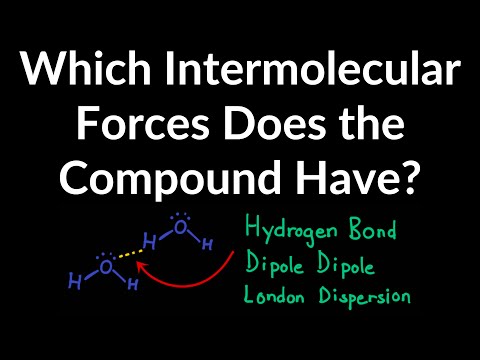
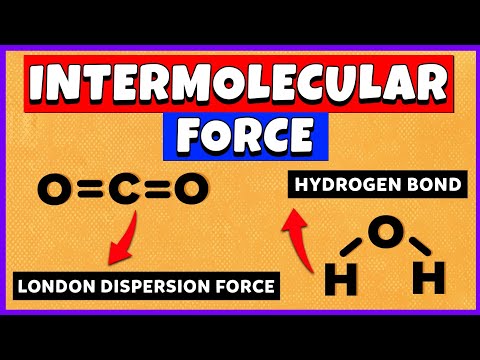
Hydrogen bonds: The second type of intermolecular force is the hydrogen bond. The permanent dipole in a covalent bond between a hydrogen atom and a fluorine, oxygen or nitrogen atom is particularly strong. Thus the attraction between the electron deficient H of one molecule and the lone pair of electrons on a fluorine, oxygen or nitrogen atom of another molecule is much stronger than the permanent dipole-dipole attraction between the two hydrogen chloride molecules. This particular type of dipole-dipole attraction between the electron deficient H of one molecule and the lone pair of electrons on a fluorine, oxygen or nitrogen atom of another molecule is given the special name of hydrogen bond.
Even though a hydrogen bond has only about 5% the strength of a covalent bond, it does have significant effects on the physical properties of compounds. Were it not for hydrogen bonds both water and alcohol would be gases at room temperature and pressure. Hydrogen bonds explain the lower volatility of alcohols compared to that of alkanes of similar molecular mass.
van der Waals’ forces: van der Waals’ forces are induced dipole-dipole interactions. They arise out of movement of the electrons in the shells.These induced dipole-dipole interactions, called van der Waals’ forces, occur in all molecules, whether polar or not, but are the only intermolecular forces between non-polar molecules such as the halogens and the noble gases. As the number of electrons in the molecule increases, so do the van der Waals’ forces.
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Learn what intermolecular forces are, the three most common types and the differences between them.
An intermolecular force is simply an attractive force between neighbouring molecules.
There are three common types of intermolecular force: permanent dipole-dipole forces, hydrogen bonds and van der Waals' forces. These three forces are much weaker than ionic or covalent bonds which bind atoms and ions together in elements and compounds.
Permanent dipole-dipole forces: A polar molecule is one in which there is a permanent dipole, arising usually because the different atoms in the molecule have different electro-negativities.
Hydrogen chloride is a polar molecule as the pair of electrons in the H---Cl bond are nearer the Cl atom because it has a greater electronegativity than the H atom. The two electrons of the covalent bond between the hydrogen and chlorine atoms are nearer the chlorine atom because of its greater electronegativity. Thus there will be an attraction between the chlorine atom of one molecule and the hydrogen atom of a neighbouring molecule.
Hydrogen bonds: The second type of intermolecular force is the hydrogen bond. The permanent dipole in a covalent bond between a hydrogen atom and a fluorine, oxygen or nitrogen atom is particularly strong. Thus the attraction between the electron deficient H of one molecule and the lone pair of electrons on a fluorine, oxygen or nitrogen atom of another molecule is much stronger than the permanent dipole-dipole attraction between the two hydrogen chloride molecules. This particular type of dipole-dipole attraction between the electron deficient H of one molecule and the lone pair of electrons on a fluorine, oxygen or nitrogen atom of another molecule is given the special name of hydrogen bond.
Even though a hydrogen bond has only about 5% the strength of a covalent bond, it does have significant effects on the physical properties of compounds. Were it not for hydrogen bonds both water and alcohol would be gases at room temperature and pressure. Hydrogen bonds explain the lower volatility of alcohols compared to that of alkanes of similar molecular mass.
van der Waals’ forces: van der Waals’ forces are induced dipole-dipole interactions. They arise out of movement of the electrons in the shells.These induced dipole-dipole interactions, called van der Waals’ forces, occur in all molecules, whether polar or not, but are the only intermolecular forces between non-polar molecules such as the halogens and the noble gases. As the number of electrons in the molecule increases, so do the van der Waals’ forces.
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Комментарии
 0:05:19
0:05:19
 0:10:54
0:10:54
 0:08:07
0:08:07
 0:10:40
0:10:40
 0:07:59
0:07:59
 0:03:38
0:03:38
 0:05:37
0:05:37
 0:08:05
0:08:05
 1:28:40
1:28:40
 0:21:14
0:21:14
 0:05:20
0:05:20
 0:06:53
0:06:53
 0:01:19
0:01:19
 0:07:36
0:07:36
 0:07:01
0:07:01
 0:15:54
0:15:54
 0:11:41
0:11:41
 0:35:58
0:35:58
 0:12:16
0:12:16
 0:01:48
0:01:48
 0:05:20
0:05:20
 0:05:50
0:05:50
 0:55:46
0:55:46
 0:03:51
0:03:51