filmov
tv
Intermolecular Forces | A-level Chemistry | OCR, AQA, Edexcel

Показать описание
Intermolecular Forces in a Snap!
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Types of Intermolecular Forces
a) London Forces
b) Permanent Dipole-Dipole Interactions
c) Hydrogen Bonds
2. The Properties Caused by these Forces
Introduction to Intermolecular Forces
Intramolecular interactions act within molecules. Atoms within molecules are held together by chemical bonds: Ionic bonds, Covalent Bonds. Intermolecular interactions are the forces of attraction between molecules. These are non-bonded interactions. They do not involve the transfer of any electrons. They are a result of the constant and random movement of electrons within the shells of atoms.
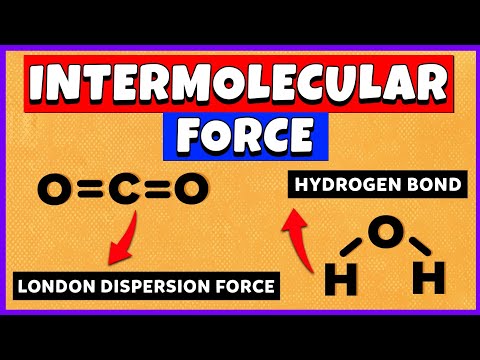
Types of Intermolecular Forces
There are 3 main types of intermolecular forces: London Forces, Permanent Dipoles, Hydrogen Bonds.
London Forces
These were proposed by German Physicist Fritz London. Rather than electrons existing statically within orbitals, they are constantly moving within the clouds of charge. On average, electron density is symmetrical. At any moment, it is likely that there are more electrons on one side of the atom than the other. Temporarily, the charge is unequally distributed. There is a temporary dipole. Like dominos, the temporarily dipole can induce a temporary dipole in a neighbouring atom. The two dipoles will be attracted to each other. These dipoles are constantly being formed and destroyed. Overall, we observe that the atoms are attracted to each other.
The Scale of London Forces
It is important to understand that London forces occur on more than an atomic scale. They occur on a molecular scale too. They occur between almost everything! In addition to other intermolecular forces of attraction.
Permanent Dipoles
We see the same interactions between molecules that have permanent dipoles. If correctly aligned, then the two molecules will attract each other. However, as the molecules are randomly and constantly moving, they do not always align. Induced dipoles are always aligned but permanent dipoles are not always aligned.
Dipole-Dipole Forces
Permanent Dipole-Permanent Dipole, Permanent Dipole-Induced Dipole.
Hydrogen Bonds
These are strong permanent dipole-permanent dipole forces of attraction. They occur between: An electron deficient hydrogen. Hydrogen bonded to a more electronegative element. Oxygen, Fluorine, Nitrogen. And the lone pair of electrons on a highly electronegative atom on another molecule.
Other Examples of Hydrogen Bonding
They occur between: An electron deficient hydrogen. Hydrogen bonded to a more electronegative element. Fluorine, Nitrogen, Oxygen. And the lone pair of electrons on a highly electronegative atom on another molecule. Example: Ammonia. All compounds containing a -N-H bond are able to form intermolecular hydrogen bonds. Example: Hydrogen Fluoride. The only flouride containing compound with intermolecular hydrogen bonding is hydrogen fluoride.
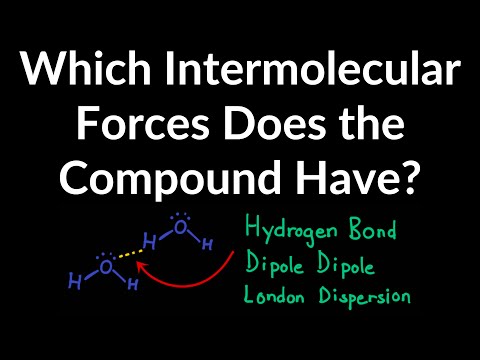
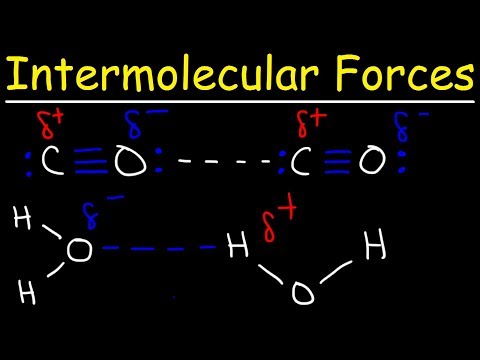
Hydrogen Bonds in Water
Hydrogen bonds exist between water molecules. This gives water some interesting properties: Ice is less dense than water. Interestingly, the solid form of water is less dense than the liquid form. Many hydrogen bonds form between water molecules in ice. These form in liquids, but break and reform easily as molecules move. The hydrogen bonds hold the water molecules apart in a fixed position. Ice has an open lattice structure or rings. High boiling and melting point. Hydrogen bonds are much stronger than other intermolecular forces. These strong intermolecular forces have to be overcome. High Surface Tension. The lattice is strong and flexible. Allows for insects to walk on water!
Types of Intermolecular Forces
The 3 main types of intermolecular forces differ in their relative strength: London Forces, Permanent Dipoles, Hydrogen Bonds.
Types of Intermolecular Forces: Boiling Points
We can see the effect of hydrogen bonding on boiling points by looking at the boiling points of hydrides of elements from Group 4-7. Trends. Noble Gases. Trend: Gradual increase in boiling poinnts. Only weak Van der Waals forces of attraction exist. Van der Waals forces of attraction increase as the number of electrons increase.
SnapRevise is the UK’s leading A-level and GCSE revision & exam preparation resource offering comprehensive video courses created by A* tutors. Our courses are designed around the OCR, AQA, SNAB, Edexcel B, WJEC, CIE and IAL exam boards, concisely covering all the important concepts required by each specification. In addition to all the content videos, our courses include hundreds of exam question videos, where we show you how to tackle questions and walk you through step by step how to score full marks.
Sign up today and together, let’s make A-level Chemistry a walk in the park!
The key points covered in this video include:
1. Types of Intermolecular Forces
a) London Forces
b) Permanent Dipole-Dipole Interactions
c) Hydrogen Bonds
2. The Properties Caused by these Forces
Introduction to Intermolecular Forces
Intramolecular interactions act within molecules. Atoms within molecules are held together by chemical bonds: Ionic bonds, Covalent Bonds. Intermolecular interactions are the forces of attraction between molecules. These are non-bonded interactions. They do not involve the transfer of any electrons. They are a result of the constant and random movement of electrons within the shells of atoms.
Types of Intermolecular Forces
There are 3 main types of intermolecular forces: London Forces, Permanent Dipoles, Hydrogen Bonds.
London Forces
These were proposed by German Physicist Fritz London. Rather than electrons existing statically within orbitals, they are constantly moving within the clouds of charge. On average, electron density is symmetrical. At any moment, it is likely that there are more electrons on one side of the atom than the other. Temporarily, the charge is unequally distributed. There is a temporary dipole. Like dominos, the temporarily dipole can induce a temporary dipole in a neighbouring atom. The two dipoles will be attracted to each other. These dipoles are constantly being formed and destroyed. Overall, we observe that the atoms are attracted to each other.
The Scale of London Forces
It is important to understand that London forces occur on more than an atomic scale. They occur on a molecular scale too. They occur between almost everything! In addition to other intermolecular forces of attraction.
Permanent Dipoles
We see the same interactions between molecules that have permanent dipoles. If correctly aligned, then the two molecules will attract each other. However, as the molecules are randomly and constantly moving, they do not always align. Induced dipoles are always aligned but permanent dipoles are not always aligned.
Dipole-Dipole Forces
Permanent Dipole-Permanent Dipole, Permanent Dipole-Induced Dipole.
Hydrogen Bonds
These are strong permanent dipole-permanent dipole forces of attraction. They occur between: An electron deficient hydrogen. Hydrogen bonded to a more electronegative element. Oxygen, Fluorine, Nitrogen. And the lone pair of electrons on a highly electronegative atom on another molecule.
Other Examples of Hydrogen Bonding
They occur between: An electron deficient hydrogen. Hydrogen bonded to a more electronegative element. Fluorine, Nitrogen, Oxygen. And the lone pair of electrons on a highly electronegative atom on another molecule. Example: Ammonia. All compounds containing a -N-H bond are able to form intermolecular hydrogen bonds. Example: Hydrogen Fluoride. The only flouride containing compound with intermolecular hydrogen bonding is hydrogen fluoride.
Hydrogen Bonds in Water
Hydrogen bonds exist between water molecules. This gives water some interesting properties: Ice is less dense than water. Interestingly, the solid form of water is less dense than the liquid form. Many hydrogen bonds form between water molecules in ice. These form in liquids, but break and reform easily as molecules move. The hydrogen bonds hold the water molecules apart in a fixed position. Ice has an open lattice structure or rings. High boiling and melting point. Hydrogen bonds are much stronger than other intermolecular forces. These strong intermolecular forces have to be overcome. High Surface Tension. The lattice is strong and flexible. Allows for insects to walk on water!
Types of Intermolecular Forces
The 3 main types of intermolecular forces differ in their relative strength: London Forces, Permanent Dipoles, Hydrogen Bonds.
Types of Intermolecular Forces: Boiling Points
We can see the effect of hydrogen bonding on boiling points by looking at the boiling points of hydrides of elements from Group 4-7. Trends. Noble Gases. Trend: Gradual increase in boiling poinnts. Only weak Van der Waals forces of attraction exist. Van der Waals forces of attraction increase as the number of electrons increase.
Комментарии
 0:10:54
0:10:54
 0:22:03
0:22:03
 0:21:25
0:21:25
 0:37:01
0:37:01
 0:14:17
0:14:17
 0:08:07
0:08:07
 0:05:30
0:05:30
 0:16:20
0:16:20
 0:05:37
0:05:37
 0:05:51
0:05:51
 0:05:27
0:05:27
 0:08:17
0:08:17
 1:05:56
1:05:56
 0:10:40
0:10:40
 0:08:05
0:08:05
 0:11:46
0:11:46
 0:03:09
0:03:09
 0:04:23
0:04:23
 0:22:10
0:22:10
 0:25:33
0:25:33
 0:03:02
0:03:02
 0:11:53
0:11:53
 0:08:08
0:08:08
 0:25:21
0:25:21