filmov
tv
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubility
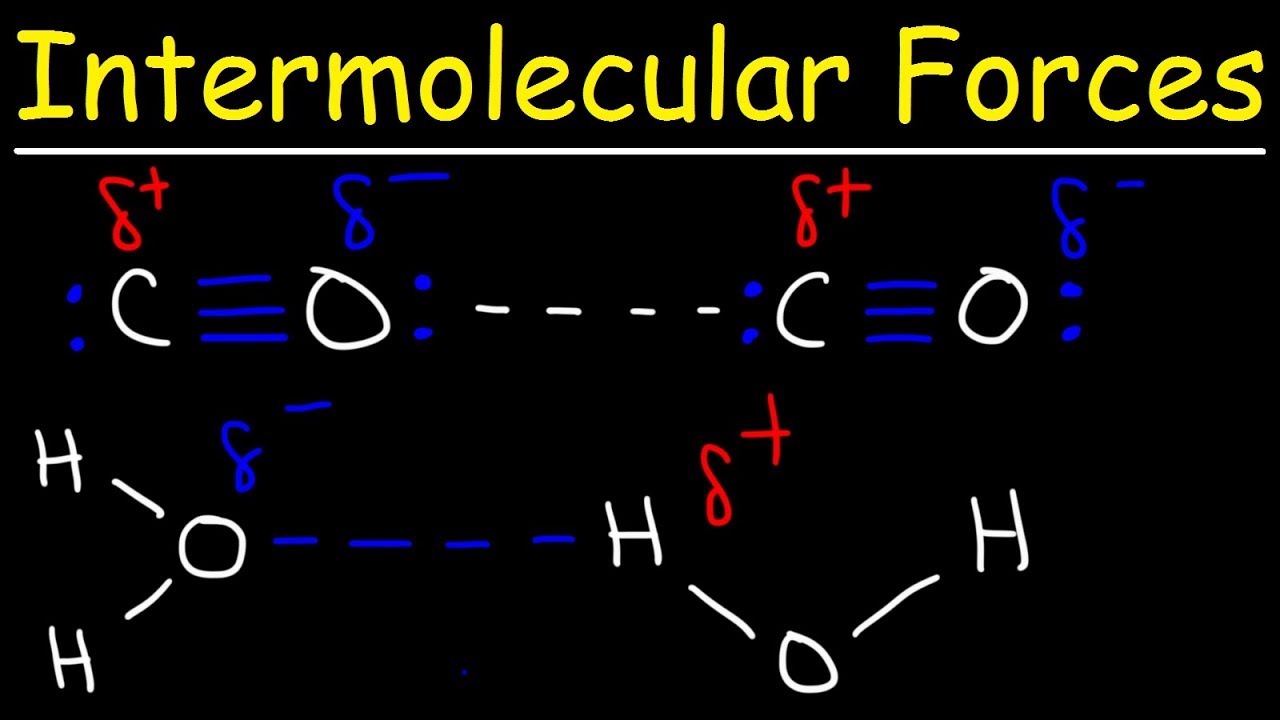
Показать описание
This organic chemistry video tutorial provides a basic introduction into intermolecular forces, hydrogen bonding, and dipole dipole interactions. It explains how to determine which molecule has a higher boiling point and which has a higher solubility in water.
Organic Chemistry - Basic Introduction:
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Steric Number and Hybridization:
Dipole Moment & Percent Ionic Character:
_______________________________
Resonance Structures:
Bond Order and Resonance:
Curve Arrow Notation:
The Major Resonance Contributor:
pKa, Ka, & Acid Strength:
Acids and Bases - Basic Intro:
_______________________________
Stability of Negative Charges:
pKa Values of Acids:
Resonance Stabilization - Conjugate Bases:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Organic Chemistry - Basic Introduction:
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Steric Number and Hybridization:
Dipole Moment & Percent Ionic Character:
_______________________________
Resonance Structures:
Bond Order and Resonance:
Curve Arrow Notation:
The Major Resonance Contributor:
pKa, Ka, & Acid Strength:
Acids and Bases - Basic Intro:
_______________________________
Stability of Negative Charges:
pKa Values of Acids:
Resonance Stabilization - Conjugate Bases:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Комментарии
 0:10:40
0:10:40
 0:06:39
0:06:39
 0:10:54
0:10:54
 0:06:47
0:06:47
 0:05:19
0:05:19
 0:06:31
0:06:31
 0:10:54
0:10:54
 0:05:37
0:05:37
 0:02:48
0:02:48
 0:08:05
0:08:05
 0:55:46
0:55:46
 0:08:05
0:08:05
 0:08:07
0:08:07
 0:07:51
0:07:51
 0:03:16
0:03:16
 0:19:29
0:19:29
 0:05:21
0:05:21
 0:00:22
0:00:22
 0:03:18
0:03:18
 0:14:17
0:14:17
 0:10:46
0:10:46
 0:05:50
0:05:50
 0:08:39
0:08:39
 0:04:55
0:04:55