filmov
tv
First Law of Thermodynamics

Показать описание
First Law of Thermodynamics
Lecture By: Er. Himanshu Vasishta, Tutorials Point India Private Limited
Lecture By: Er. Himanshu Vasishta, Tutorials Point India Private Limited
The First Law of Thermodynamics: Internal Energy, Heat, and Work
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
FIRST LAW OF THERMODYNAMICS | Easy and Short
What is the First Law of Thermodynamics?
Thermodynamics: Crash Course Physics #23
First law of thermodynamics class 11 nbf | 11th class physics ch 10 | kpk, federal, punjab board
First Law of Thermodynamics, Basic Introduction, Physics Problems
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
The First Law of Thermodynamics | Thermodynamics | (Solved Examples)
First law of Thermodynamics | Physics
First Law of Thermodynamics
First Law of Thermodynamics - Concept and Examples
First law of thermodynamics | class 11 physics | Thermodynamics | BS physics| BSc | physics ka safar
Simplifying the First Law of Thermodynamics | Physics by Parth G
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
Ch#11 |Lec#6 | First Law of thermodynamics and Applications |Law of conservation of energy #chem11
First Law of Thermodynamics
First law of thermodynamics | Chemical Processes | MCAT | Khan Academy
First Law of Thermodynamics introduction | Biology | Khan Academy
First Law of Thermodynamics
First law of thermodynamics || class 11/12 physics
5.1 First Law of Thermodynamics and Enthalpy | General Chemistry
2.1. 1st Law of Thermodynamics
First Law of Thermodynamics
Комментарии
 0:05:44
0:05:44
 0:11:27
0:11:27
 0:02:09
0:02:09
 0:04:09
0:04:09
 0:10:04
0:10:04
 0:26:30
0:26:30
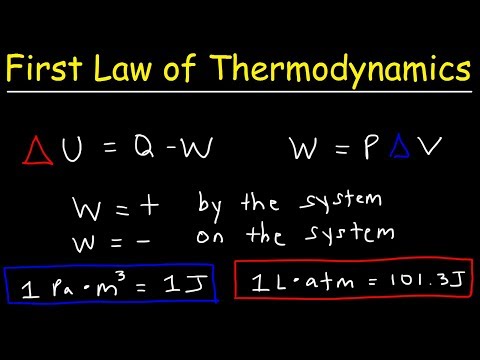 0:10:31
0:10:31
 0:01:53
0:01:53
 0:09:52
0:09:52
 0:11:41
0:11:41
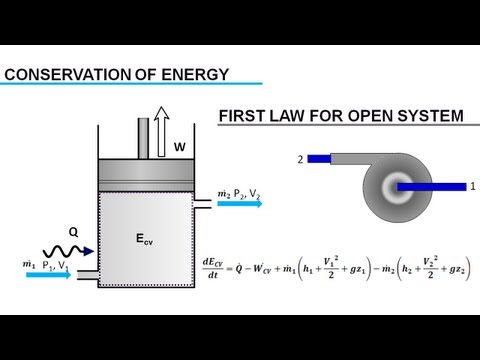 0:06:34
0:06:34
 0:03:29
0:03:29
 0:35:23
0:35:23
 0:07:39
0:07:39
 0:10:05
0:10:05
 0:13:42
0:13:42
 0:04:10
0:04:10
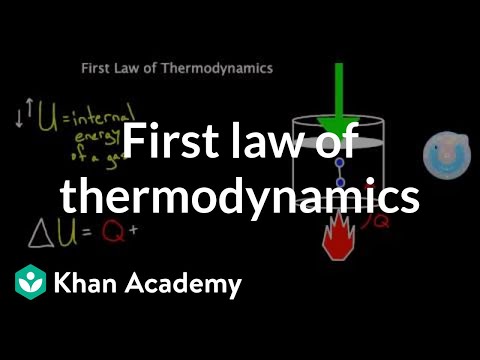 0:11:23
0:11:23
 0:09:17
0:09:17
 1:14:06
1:14:06
 0:22:44
0:22:44
 0:29:59
0:29:59
 3:12:15
3:12:15
 0:03:14
0:03:14