filmov
tv
2.1. 1st Law of Thermodynamics

Показать описание
Lecture on the first law of thermodynamics and its applications in ideal gas processes and thermochemistry.
Outline:
0:32 INTRODUCTION: Definition of Thermodynamics
2:33 System and Surroundings
4:16 Extensive vs. Intensive Properties
7:14 Definition of energy
16:34 Statement of the First Law of Thermodynamics
17:19 State vs. Non-state functions
23:49 Work: pressure-volume work, example of work as isothermal irreversible and reversible PV work
54:14 Heat
56:16 Heat Capacity
1:12:30 IDEAL GAS PROCESSES
1:13:19 Isochoric Process
1:17:37 Isobaric Process
1:25:35 Definition of Enthalpy
1:29:55 Cp vs Cv
1:31:43 Cp and Cv of monatomic and diatomic gases
1:35:14 Isothermal Process: irreversible and reversible
1:44:31 Adiabatic Process: irreversible and reversible
2:12:04 Summary of Ideal Gas Processes
2:29:52 THERMOCHEMSITRY
2:33:30 Relationship between enthalpy and internal energy
2:38:14 Calorimetry
2:44:54 Hess's Law
2:51:13 Temperature Dependence of Enthalpy Changes: Phase Changes, Chemical Changes and Kirchoff's Rule
Outline:
0:32 INTRODUCTION: Definition of Thermodynamics
2:33 System and Surroundings
4:16 Extensive vs. Intensive Properties
7:14 Definition of energy
16:34 Statement of the First Law of Thermodynamics
17:19 State vs. Non-state functions
23:49 Work: pressure-volume work, example of work as isothermal irreversible and reversible PV work
54:14 Heat
56:16 Heat Capacity
1:12:30 IDEAL GAS PROCESSES
1:13:19 Isochoric Process
1:17:37 Isobaric Process
1:25:35 Definition of Enthalpy
1:29:55 Cp vs Cv
1:31:43 Cp and Cv of monatomic and diatomic gases
1:35:14 Isothermal Process: irreversible and reversible
1:44:31 Adiabatic Process: irreversible and reversible
2:12:04 Summary of Ideal Gas Processes
2:29:52 THERMOCHEMSITRY
2:33:30 Relationship between enthalpy and internal energy
2:38:14 Calorimetry
2:44:54 Hess's Law
2:51:13 Temperature Dependence of Enthalpy Changes: Phase Changes, Chemical Changes and Kirchoff's Rule
The First Law of Thermodynamics: Internal Energy, Heat, and Work
FIRST LAW OF THERMODYNAMICS | Easy and Short
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
2.1. 1st Law of Thermodynamics
The First Law of Thermodynamics | Thermodynamics | (Solved Examples)
Thermodynamics - 2-6 The first law of thermodynamics (conservation of energy)
Thermodynamics: Crash Course Physics #23
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
UG 3rd SEM : Physics(E/M)
Simplifying the First Law of Thermodynamics | Physics by Parth G
First law of thermodynamics class 11 nbf | 11th class physics ch 10 | kpk, federal, punjab board
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
First Law of Thermodynamics, Basic Introduction, Physics Problems
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
The First Law of Thermodynamics — The Balance of Energy — Lesson 1, Part 2
First law of Thermodynamics | Physics
Understanding Second Law of Thermodynamics !
The Zeroth Law of Thermodynamics: Thermal Equilibrium
What is 1st law of thermodynamics #thermodynamics #1stlawofthermodynamics #heat
First law of thermodynamics || class 11/12 physics
What is the Difference Between First & Second Law of Thermodynamics | Physics
18.1 The Laws of Thermodynamics | General Chemistry
First Law of Thermodynamics
Комментарии
 0:05:44
0:05:44
 0:02:09
0:02:09
 0:11:27
0:11:27
 3:12:15
3:12:15
 0:09:52
0:09:52
 0:07:42
0:07:42
 0:10:04
0:10:04
 0:01:53
0:01:53
 0:53:54
0:53:54
 0:07:39
0:07:39
 0:26:30
0:26:30
 0:08:12
0:08:12
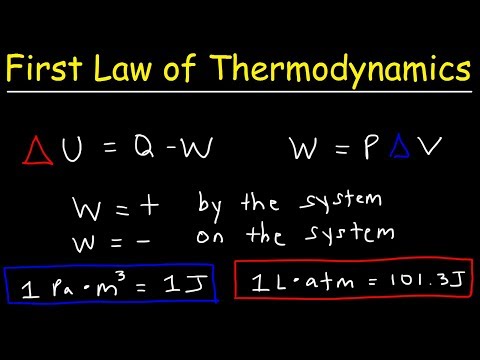 0:10:31
0:10:31
 0:04:11
0:04:11
 0:10:05
0:10:05
 0:12:30
0:12:30
 0:11:41
0:11:41
 0:06:56
0:06:56
 0:03:29
0:03:29
 0:00:33
0:00:33
 0:22:44
0:22:44
 0:01:51
0:01:51
 0:10:06
0:10:06
 0:00:53
0:00:53