filmov
tv
5.1 First Law of Thermodynamics and Enthalpy | General Chemistry
Показать описание
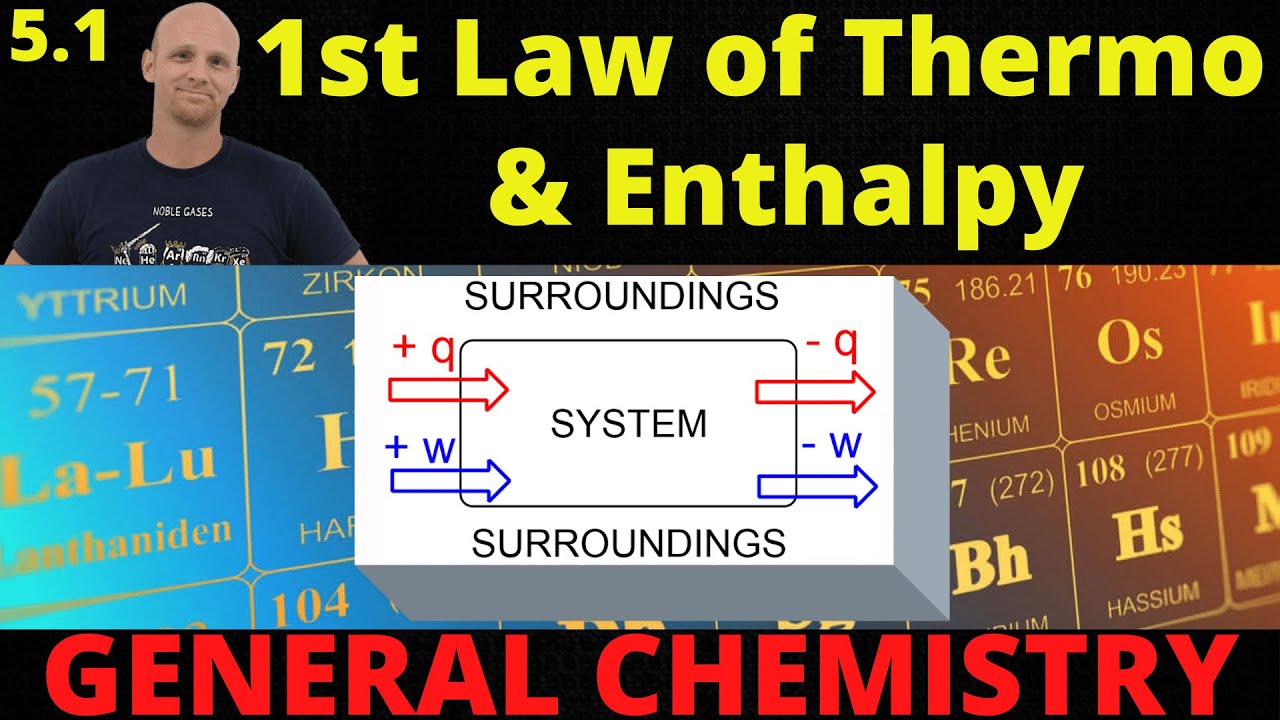
Chad introduces the topic of energy and its units, comprehensively covers the First Law of Thermodynamics, and introduces Enthalpy. First, a basic definition of energy is provided and the Joules, calories, and nutritional Calories are introduced. The First Law of Thermodynamics is then covered both qualitatively and quantitatively as described by the equation:
DE = q + w
Calculations involving the flow of energy between the system and surroundings through heat and work are explored.
Enthalpy is then introduced with a basic definition along with the definitions of Endothermic and Exothermic as types of reactions. An application of enthalpy to stoichiometry (thermochemical stoichiometry) then follows, and the lesson is concluded by describing/defining the six fundamental phase changes and the enthalpy associated with each.
00:00 Lesson Introduction
01:04 Energy, Joules, and Calories
02:48 First Law of Thermodynamics
11:23 Enthalpy
19:26 Enthalpy Stoichiometry
23:29 Enthalpy and Phase Changes
DE = q + w
Calculations involving the flow of energy between the system and surroundings through heat and work are explored.
Enthalpy is then introduced with a basic definition along with the definitions of Endothermic and Exothermic as types of reactions. An application of enthalpy to stoichiometry (thermochemical stoichiometry) then follows, and the lesson is concluded by describing/defining the six fundamental phase changes and the enthalpy associated with each.
00:00 Lesson Introduction
01:04 Energy, Joules, and Calories
02:48 First Law of Thermodynamics
11:23 Enthalpy
19:26 Enthalpy Stoichiometry
23:29 Enthalpy and Phase Changes
The First Law of Thermodynamics: Internal Energy, Heat, and Work
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
5.1 First Law of Thermodynamics and Enthalpy | General Chemistry
FIRST LAW OF THERMODYNAMICS | Easy and Short
What is the First Law of Thermodynamics?
Simplifying the First Law of Thermodynamics | Physics by Parth G
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Energy Rates Example of First Law of Thermodynamics in 3 Minutes!
Zeroth Law of Thermodynamics: The Foundation of Temperature and Equilibrium
First Law of Thermodynamics, Basic Introduction, Physics Problems
Thermodynamics: Crash Course Physics #23
Thermodynamics - A-level Physics
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
First law of thermodynamics class 11 nbf | 11th class physics ch 10 | kpk, federal, punjab board
First Law of Thermodynamics [year-1]
First law of Thermodynamics | Physics
What is Thermodynamics? | Class 11 Physics Explained
First Law of Thermodynamics
Energy Conservation Equation & FIRST LAW of Thermodynamics in 12 Minutes!
18.1 The Laws of Thermodynamics | General Chemistry
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
ZEROTH LAW OF THERMODYNAMICS | Simple & Basic Animation
First Law of Thermodynamics | History & Background | Proof | laws of thermodynamics explained si...
Mechanical Engineering Thermodynamics - Lec 5, pt 1 of 3: First Law - Open System - Unsteady Flow
Комментарии
 0:05:44
0:05:44
 0:11:27
0:11:27
 0:29:59
0:29:59
 0:02:09
0:02:09
 0:04:09
0:04:09
 0:07:39
0:07:39
 0:08:12
0:08:12
 0:02:38
0:02:38
 0:00:16
0:00:16
 0:10:31
0:10:31
 0:10:04
0:10:04
 0:12:33
0:12:33
 0:01:53
0:01:53
 0:26:30
0:26:30
 0:08:40
0:08:40
 0:11:41
0:11:41
 0:00:53
0:00:53
 0:03:14
0:03:14
 0:12:00
0:12:00
 0:10:06
0:10:06
 0:10:05
0:10:05
 0:02:40
0:02:40
 0:16:09
0:16:09
 0:10:04
0:10:04