filmov
tv
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry

Показать описание
This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. It shows the relationship between internal energy, heat, and work. It discusses the difference between the system and surroundings and distinguishes an open system from a closed system and an isolated system. In chemistry, work is positive whenever work is done on the system and work is negative whenever work is done by the system. During an exothermic process, heat flows out of the system and it flows into the system during an endothermic process.
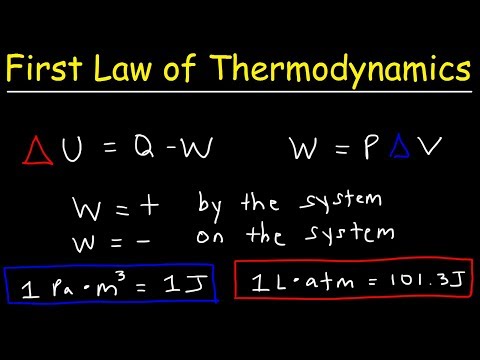
First Law of Thermodynamics:
Thermochemistry Equations:
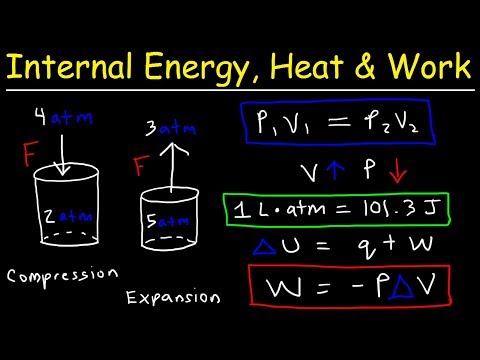
Internal Energy, Heat, and Work:
Thermochemical Equations:
Specific Vs Molar Heat Capacity:
________________________________
Basic Calorimetry Problems:
Final Temperature Calorimetry Problems:
Latent Heat of Fusion & Vaporization:
Coffee Cup Calorimeter:
More Calorimeter Problems:
__________________________________
Specific Heat Capacity Problems:
Hess Law Problems:
More Hess Law Problems:
Enthalpy of Formation & Heat Combustion:
Enthalpy Practice Problems:
__________________________________
Speed of Light, Frequency, & Wavelength:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
First Law of Thermodynamics:
Thermochemistry Equations:
Internal Energy, Heat, and Work:
Thermochemical Equations:
Specific Vs Molar Heat Capacity:
________________________________
Basic Calorimetry Problems:
Final Temperature Calorimetry Problems:
Latent Heat of Fusion & Vaporization:
Coffee Cup Calorimeter:
More Calorimeter Problems:
__________________________________
Specific Heat Capacity Problems:
Hess Law Problems:
More Hess Law Problems:
Enthalpy of Formation & Heat Combustion:
Enthalpy Practice Problems:
__________________________________
Speed of Light, Frequency, & Wavelength:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:11:27
0:11:27
 0:05:44
0:05:44
 0:10:31
0:10:31
 0:02:09
0:02:09
 0:04:09
0:04:09
 0:10:04
0:10:04
 0:06:34
0:06:34
 0:03:29
0:03:29
 0:38:46
0:38:46
 0:10:05
0:10:05
 0:04:10
0:04:10
 0:08:12
0:08:12
 0:08:40
0:08:40
 0:07:39
0:07:39
 0:11:41
0:11:41
 0:01:53
0:01:53
 0:07:01
0:07:01
 0:09:52
0:09:52
 0:06:56
0:06:56
 0:04:39
0:04:39
 0:52:48
0:52:48
 0:23:29
0:23:29
 0:03:41
0:03:41
 0:04:11
0:04:11