filmov
tv
First Law of Thermodynamics, Basic Introduction, Physics Problems

Показать описание
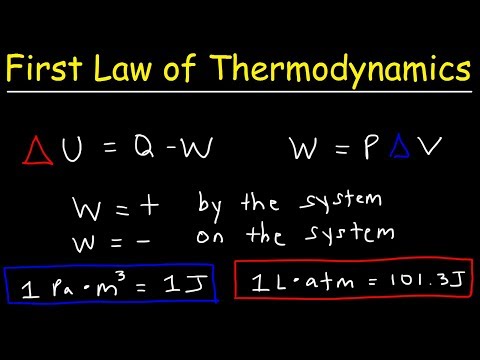
This physics video tutorial provides a basic introduction into the first law of thermodynamics which is associated with the law of conservation of energy. There are two main ways to change the internal energy of a system - that is, through heat and mechanical work. This thermodynamics video tutorial contains plenty examples and practice problems on heat, work, and internal energy calculations.
Physics Video Lessons:
_________________________________
Open Vs Closed Vs Isolated System:
Isobaric Process:
Isochoric Process:
Isothermal Process:
Internal Energy of an Ideal Gas:
_________________________
Adiabatic Process:
PV Diagrams:
Thermodynamics Review:
2nd Law of Thermodynamics:
Heat Engines:
Converting Heat Into Electricity:
________________________
Carnot Cycle:
Otto Cycle:
Refrigerators and Heat Pumps:
Entropy:
Heat Engines and Refrigerators Review:
Physics PDF Worksheets:
Physics Video Lessons:
_________________________________
Open Vs Closed Vs Isolated System:
Isobaric Process:
Isochoric Process:
Isothermal Process:
Internal Energy of an Ideal Gas:
_________________________
Adiabatic Process:
PV Diagrams:
Thermodynamics Review:
2nd Law of Thermodynamics:
Heat Engines:
Converting Heat Into Electricity:
________________________
Carnot Cycle:
Otto Cycle:
Refrigerators and Heat Pumps:
Entropy:
Heat Engines and Refrigerators Review:
Physics PDF Worksheets:
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
The First Law of Thermodynamics: Internal Energy, Heat, and Work
FIRST LAW OF THERMODYNAMICS | Easy and Short
First Law of Thermodynamics, Basic Introduction, Physics Problems
What is the First Law of Thermodynamics?
Thermodynamics: Crash Course Physics #23
First Law of Thermodynamics - Concept and Examples
First Law of Thermodynamics.
First law of Thermodynamics | Physics
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
First law of thermodynamics class 11 nbf | 11th class physics ch 10 | kpk, federal, punjab board
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Ch#11 |Lec#6 | First Law of thermodynamics and Applications |Law of conservation of energy #chem11
First law of Thermodynamics (Animation)
Engineering Thermodynamics/ zeroth, first, second and third law of thermodynamics/explained in tamil
First Law of Thermodynamics (Basics, Significance, Applications & Limitations) Explained
Understanding Second Law of Thermodynamics !
The Zeroth Law of Thermodynamics: Thermal Equilibrium
First Law of Thermodynamics
First Law of Thermodynamics
First Law of Thermodynamics
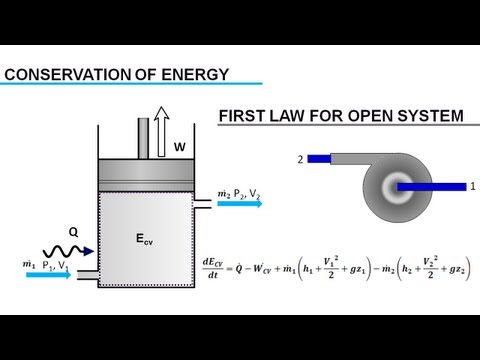
5.1 First Law of Thermodynamics and Enthalpy | General Chemistry
The First Law of Thermodynamics | Thermodynamics | (Solved Examples)
Комментарии
 0:11:27
0:11:27
 0:05:44
0:05:44
 0:02:09
0:02:09
 0:10:31
0:10:31
 0:04:09
0:04:09
 0:10:04
0:10:04
 0:03:29
0:03:29
 0:00:29
0:00:29
 0:11:41
0:11:41
 0:01:53
0:01:53
 0:10:05
0:10:05
 0:26:30
0:26:30
 0:08:12
0:08:12
 0:13:42
0:13:42
 0:07:01
0:07:01
 0:12:08
0:12:08
 0:09:17
0:09:17
 0:06:56
0:06:56
 0:03:29
0:03:29
 0:06:34
0:06:34
 0:00:53
0:00:53
 1:14:06
1:14:06
 0:29:59
0:29:59
 0:09:52
0:09:52