filmov
tv
The First Law of Thermodynamics: Internal Energy, Heat, and Work

Показать описание
In chemistry we talked about the first law of thermodynamics as being the law of conservation of energy, and that's one way of looking at it, but physicists like doing math more than chemists do, so let's talk about the first law in terms of an equation. You like equations by now, don't you? You should!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
The First Law of Thermodynamics: Internal Energy, Heat, and Work
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
FIRST LAW OF THERMODYNAMICS | Easy and Short
What is the First Law of Thermodynamics?
First Law of Thermodynamics, Basic Introduction, Physics Problems
First Law of Thermodynamics
Thermodynamics: Crash Course Physics #23
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
Applications of First Law of Thermodynamics - Unit 16 First Law of Thermodynamics
Simplifying the First Law of Thermodynamics | Physics by Parth G
First law of Thermodynamics (Animation)
First Law of Thermodynamics - Concept and Examples
The First Law of Thermodynamics | Thermodynamics | (Solved Examples)
First law of Thermodynamics | Physics
5.1 First Law of Thermodynamics and Enthalpy | General Chemistry
First Law of Thermodynamics [year-1]
First Law of Thermodynamics
Thermodynamics - 2-6 The first law of thermodynamics (conservation of energy)
Thermodynamics - A-level Physics
First law of thermodynamics class 11 | 11th class physics ch 10 | kpk, federal, punjab board
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Ch#11 |Lec#6 | First Law of thermodynamics and Applications |Law of conservation of energy #chem11
First law of thermodynamics problem solving | Chemical Processes | MCAT | Khan Academy
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Комментарии
 0:05:44
0:05:44
 0:11:27
0:11:27
 0:02:09
0:02:09
 0:04:09
0:04:09
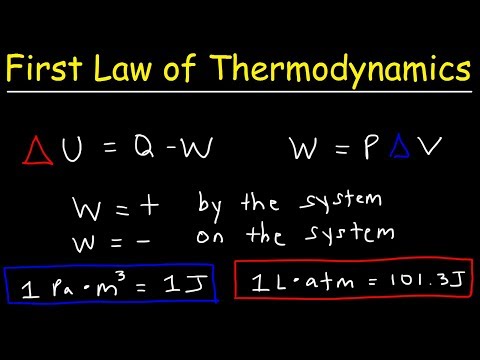 0:10:31
0:10:31
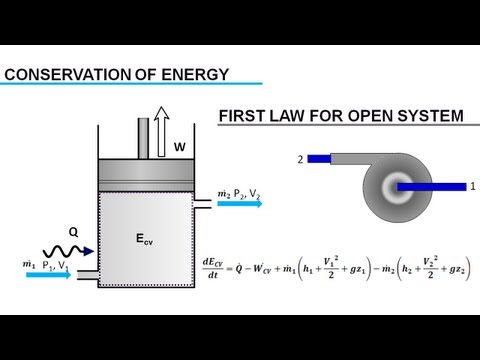 0:06:34
0:06:34
 0:10:04
0:10:04
 0:10:05
0:10:05
 0:38:46
0:38:46
 0:07:39
0:07:39
 0:07:01
0:07:01
 0:03:29
0:03:29
 0:09:52
0:09:52
 0:11:41
0:11:41
 0:29:59
0:29:59
 0:08:40
0:08:40
 0:04:10
0:04:10
 0:07:42
0:07:42
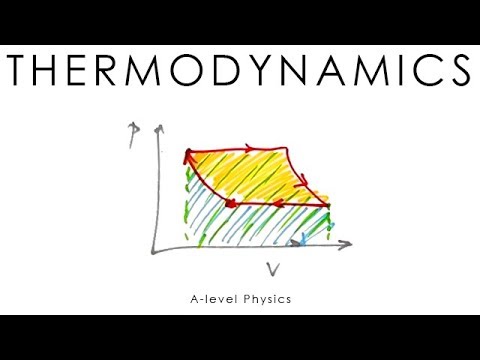 0:12:33
0:12:33
 0:26:30
0:26:30
 0:01:53
0:01:53
 0:13:42
0:13:42
 0:07:34
0:07:34
 0:08:12
0:08:12