filmov
tv
Applications of First Law of Thermodynamics - Unit 16 First Law of Thermodynamics

Показать описание
This video includes.
Boards: Sindh Boards
Class : 12
Subject: Physics
Unit: 16 First Law of Thermodynamics
Topic:
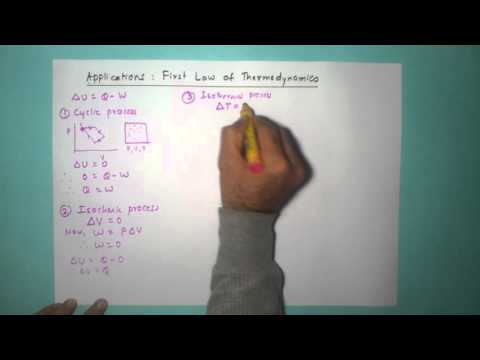
1. Statement of First law of thermodynamics
2. Applications of first law of thermodynamics in
(i) Isobaric process (ii) Isochoric Process (iii) Iso thermal process (iv) Adiabatic Process
3. Examples and graph of all applications
Boards: Sindh Boards
Class : 12
Subject: Physics
Unit: 16 First Law of Thermodynamics
Topic:
1. Statement of First law of thermodynamics
2. Applications of first law of thermodynamics in
(i) Isobaric process (ii) Isochoric Process (iii) Iso thermal process (iv) Adiabatic Process
3. Examples and graph of all applications
The First Law of Thermodynamics: Internal Energy, Heat, and Work
Applications Of First Law Of Thermodynamics- Work- Thermodynamics (Part 6)
Inertia: Newton’s First Law
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
First Law of Thermodynamics - Concept and Examples
FIRST LAW OF THERMODYNAMICS | Easy and Short
First law of thermodynamics Applications
Applications of newton's first law of motion in | Physics
Applications of Bernoulli's Equation: Atomizer, Filter Pump, Venturi Meter, Torricelli's T...
First Law of Thermodynamics Explained: Basics, Limitations, and Applications
applications of first law of thermodynamics
First law of Thermodynamics | Law of conservation of energy | Applications | Thermodynamics
Ch#11 |Lec#6 | First Law of thermodynamics and Applications |Law of conservation of energy #chem11
First law of Thermodynamics (Animation)
Application of first law of thermodynamics class 11 | Isothermal, Isobaric, Isochoric, Adiabatic
Applications of Newton's First Law
Newton's First (1st) Law of Motion with daily life examples
Applications of First Order Differential Equations - Newton's Law of Cooling
Day-To-Day Applications of First Law of Thermodynamics.
Newton's First Law of Motion | Forces and Motion | Physics | Infinity Learn
First Law of Thermodynamics (Basics, Significance, Applications & Limitations) Explained
Newton's First Law of Motion
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Thermodynamics-4 | Applications of First law of thermodynamics | Class 11
Комментарии
 0:05:44
0:05:44
 0:13:56
0:13:56
 0:02:51
0:02:51
 0:11:27
0:11:27
 0:03:29
0:03:29
 0:02:09
0:02:09
 0:07:48
0:07:48
 0:07:27
0:07:27
 0:13:37
0:13:37
 0:06:54
0:06:54
 0:10:45
0:10:45
 0:02:48
0:02:48
 0:13:42
0:13:42
 0:07:01
0:07:01
 0:51:40
0:51:40
 0:02:54
0:02:54
 0:02:21
0:02:21
 0:08:25
0:08:25
 0:04:59
0:04:59
 0:02:32
0:02:32
 0:09:17
0:09:17
 0:13:57
0:13:57
 0:01:53
0:01:53
 0:18:09
0:18:09