filmov
tv
What Are Acids & Bases? | Chemistry Basics

Показать описание
Acids and bases can be found all throughout your everyday life and are often times very useful… even in your own body! But what is it about acids that make some super dangerous and other safe to eat? And in order to tell how acidic or basic a chemical is, how do we measure their individual intensities? This episode of chemistry basics covers the fundamentals of acids and bases and how we use the pH scale to measure them.
You might also like:
ChemMatters:
Middle School Chemistry – Lesson Overviews:
Kids Chemistry:
Produced by the American Chemical Society, the world’s largest scientific society. ACS is a global leader in providing access to chemistry-related information and research through its multiple databases, peer-reviewed journals and scientific conferences.
You might also like:
ChemMatters:
Middle School Chemistry – Lesson Overviews:
Kids Chemistry:
Produced by the American Chemical Society, the world’s largest scientific society. ACS is a global leader in providing access to chemistry-related information and research through its multiple databases, peer-reviewed journals and scientific conferences.
GCSE Chemistry - Acids and Bases #34
What is a base in Chemistry? Acids and Bases
What Are Acids & Bases? | Chemistry Basics
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton
Acid and Base | Acids, Bases & pH | Video for Kids
Acids and Bases, pH and pOH
Acids and Bases - Basic Introduction - Chemistry
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained
Acids bases and salts Class 10th
General Chemistry | Acids & Bases
Acids and Bases for Kids | Learn the difference between an acid and a base
Acids, Bases, and pH
Acids and Bases and Salts - Introduction | Chemistry | Infinity Learn
Acids Bases and Salts
Properties of Acids and Bases | The Basics
Acids + Bases Made Easy! Part 1 - What the Heck is an Acid or Base? - Organic Chemistry
Acid-Base Reactions in Solution: Crash Course Chemistry #8
Acids, Bases and pH
Acids and Bases - Basic Introduction - Organic Chemistry
Acid Base Disorders and ABG Interpretation | Introduction
pH and pOH: Crash Course Chemistry #30
Chemistry Lesson | pH | Acid & Base Explained | Science for Kids
Acids and Bases - Introduction | Acid Bases and Salts | Don't Memorise
pH Scale in Simple Terms
Комментарии
 0:04:37
0:04:37
 0:02:43
0:02:43
 0:03:01
0:03:01
 0:03:48
0:03:48
 0:03:13
0:03:13
 0:09:01
0:09:01
 0:58:42
0:58:42
 0:06:01
0:06:01
 0:00:29
0:00:29
 0:33:17
0:33:17
 0:09:23
0:09:23
 0:08:54
0:08:54
 0:04:25
0:04:25
 0:17:58
0:17:58
 0:02:25
0:02:25
 0:04:57
0:04:57
 0:11:17
0:11:17
 0:02:02
0:02:02
 0:29:55
0:29:55
 0:58:08
0:58:08
 0:11:23
0:11:23
 0:03:10
0:03:10
 0:01:54
0:01:54
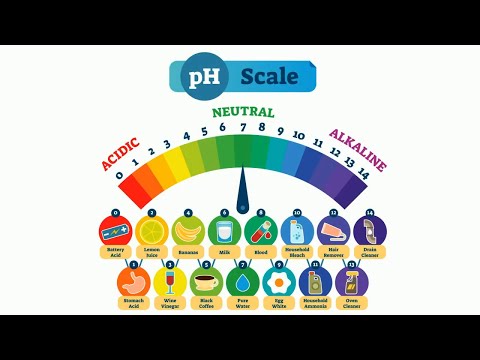 0:03:09
0:03:09