filmov
tv
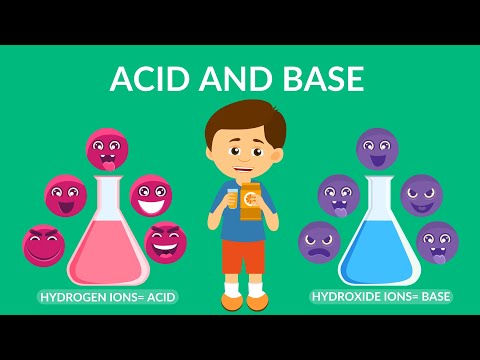
What is a base in Chemistry? Acids and Bases

Показать описание
Learn the difference between an acid and a base.The acidity of a solution is measured by its number of hydrogen ions. You can look up this measure on a ph scale. The abbreviation pH stands for potential hydrogen, and it is a measure how much hydrogen is in the solution and how active the hydrogen ion is. Hydrogen ions determine whether a solution is an acid or a base
A base that is water-soluble is called an alkali.
Acids are hydrogen ion donors and alkalis are hydrogen receptors.
A base that is water-soluble is called an alkali.
Acids are hydrogen ion donors and alkalis are hydrogen receptors.
Acid and Base | Acids, Bases & pH | Video for Kids
What is the Safest Base Possible in Minecraft?
a base-neutral system for naming numbering systems
Chemistry Lesson | pH | Acid & Base Explained | Science for Kids
Acid-Base Titration
Acid and Base Definitions | Arrhenius, Bronsted-Lowry, and Lewis
Why a NATO base in Germany could be targeted for sabotage | DW News
Acid-Base Reactions in Solution: Crash Course Chemistry #8
How a base survived 8 years on 2b2t
Acid-Base Equilibria and Buffer Solutions
Why There's a CIA Base in the Center of Australia
Acid-Base Equilibrium
Hidden base in a hidden base in a hidden base in a hidden base in a hidden base in a hidden base...
How the UNFINDABLE Minecraft 2b2t Base... was Found
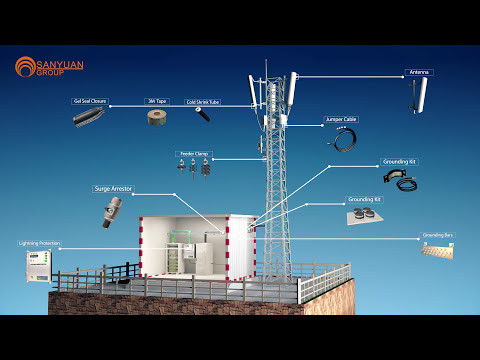
Telecom Base Station Materials: A 3D Walkthrough
wikiHow: How to Take Down a Base in Nerf
Choosing A Base in Project Zomboid! How To Choose The Best Base Location in Project Zomboid!
Making Minecraft's Most Hidden Base
How This Base Survived 12 Years on 2b2t
Every US Military Base, Mapped
How To Build A Secret Base! [Easy]
What Is The Difference Between An Airline Base And A Hub?
Acid/Base Dissociation Constant
Clash of Clans Beginner Tip: 28 Second Farming Base Search Trick
Комментарии
 0:03:13
0:03:13
 0:13:21
0:13:21
 0:17:45
0:17:45
 0:03:10
0:03:10
 0:02:40
0:02:40
 0:02:05
0:02:05
 0:10:59
0:10:59
 0:11:17
0:11:17
 0:10:41
0:10:41
 0:05:04
0:05:04
 0:11:30
0:11:30
 0:10:27
0:10:27
 0:16:07
0:16:07
 0:10:35
0:10:35
 0:02:31
0:02:31
 0:12:00
0:12:00
 0:06:16
0:06:16
 0:13:43
0:13:43
 0:10:07
0:10:07
 0:16:16
0:16:16
 0:04:07
0:04:07
 0:05:00
0:05:00
 0:07:50
0:07:50
 0:00:22
0:00:22