filmov
tv
Properties of Acids and Bases | The Basics

Показать описание
Learn all the basics about acids and bases including pH, salt formation, and complete with examples of acids and bases.
Thanks for stopping by, this is 2 minute classroom and today we are talking about acids and bases which are fascinating.
If your looking for the Arrhenius and Bronsted-Lowry definitions of an acid and base, I discuss that in a separate video, linked here and in the description.
Acids and Bases are both corrosive substances that react with and damage or destroy other substances they come in contact with.
Acids can burn the skin and cause pain, usually have a sour taste, can corrode metals, and turn blue litmus paper red.
Bases, on the other hand, have a bitter taste, feel slippery/soapy to touch, and turn red litmus paper blue.
The strength of an acid or base is measured with using the pH scale. This scale ranges from 0-14. The lower the number, the more acidic the substance, and the higher the number the less acidic or more basic and alkaline the substance. 7 is considered neutral, and pure H2O has a pH of 7.
It is possible to have a pH greater than 14 or less than 0, but you won’t come across those very often.
An example of a weak acid is carbonic acid which has a pH of 4 and can be found in carbonated beverages. Hydrochloric acid has a pH of 1 and is a strong acid, this is the acid in your stomach that helps with digestion.
An example of a weak base is calcium carbonate which has a pH of 10 and can be found in antacids, like tums. Sodium hydroxide has a pH of 14 and is a strong base, and can be found in some drain cleaners.
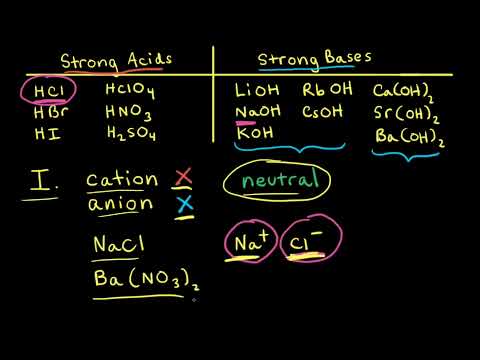
Strong acids and strong bases can be combined to for form a salt and H2O. For example, combining HCl and NaOH forms NaCl and H2O.
Most people know that strong acids can be dangerous, but strong bases can also cause harm as well. Be sure to take the necessary safety steps when handling such chemicals to avoid any injury.
If you learned something in the video, do me a favor and hit that like button. I you have more questions or comments I would love to hear them.
Don’t forget to watch my other videos and I’ll catch you next time.
Thanks for stopping by, this is 2 minute classroom and today we are talking about acids and bases which are fascinating.
If your looking for the Arrhenius and Bronsted-Lowry definitions of an acid and base, I discuss that in a separate video, linked here and in the description.
Acids and Bases are both corrosive substances that react with and damage or destroy other substances they come in contact with.
Acids can burn the skin and cause pain, usually have a sour taste, can corrode metals, and turn blue litmus paper red.
Bases, on the other hand, have a bitter taste, feel slippery/soapy to touch, and turn red litmus paper blue.
The strength of an acid or base is measured with using the pH scale. This scale ranges from 0-14. The lower the number, the more acidic the substance, and the higher the number the less acidic or more basic and alkaline the substance. 7 is considered neutral, and pure H2O has a pH of 7.
It is possible to have a pH greater than 14 or less than 0, but you won’t come across those very often.
An example of a weak acid is carbonic acid which has a pH of 4 and can be found in carbonated beverages. Hydrochloric acid has a pH of 1 and is a strong acid, this is the acid in your stomach that helps with digestion.
An example of a weak base is calcium carbonate which has a pH of 10 and can be found in antacids, like tums. Sodium hydroxide has a pH of 14 and is a strong base, and can be found in some drain cleaners.
Strong acids and strong bases can be combined to for form a salt and H2O. For example, combining HCl and NaOH forms NaCl and H2O.
Most people know that strong acids can be dangerous, but strong bases can also cause harm as well. Be sure to take the necessary safety steps when handling such chemicals to avoid any injury.
If you learned something in the video, do me a favor and hit that like button. I you have more questions or comments I would love to hear them.
Don’t forget to watch my other videos and I’ll catch you next time.
Комментарии
 0:02:25
0:02:25
 0:04:37
0:04:37
 0:58:42
0:58:42
 0:09:23
0:09:23
 0:09:01
0:09:01
 0:02:43
0:02:43
 0:04:12
0:04:12
 0:12:56
0:12:56
 0:33:17
0:33:17
 0:13:33
0:13:33
 0:08:54
0:08:54
 0:03:13
0:03:13
 0:04:25
0:04:25
 0:17:58
0:17:58
 0:09:23
0:09:23
 0:06:49
0:06:49
 0:07:42
0:07:42
 0:04:42
0:04:42
 0:15:26
0:15:26
 0:05:22
0:05:22
 0:13:50
0:13:50
 0:07:33
0:07:33
 0:09:20
0:09:20
 0:00:49
0:00:49