filmov
tv
Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy |Crash Chemistry Academy

Показать описание
A crash course tutorial on atomic orbitals, quantum numbers and electron configurations + practice problems explained.
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
-Stoichiometry Tutorial, step by step
-Types of Chemical Reactions: How to classify five basic reaction types
-Solution Stoichiometry
-Orbitals the Basics: Atomic Orbitals Tutorial
-Hybrid Orbitals Explained
-Polar Molecules Tutorial: How to determine polarity in a molecule
-Metallic Bonding and Metallic Properties Explained
-Covalent Bonding Tutorial
-Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
-Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
-Metric Unit Prefix Conversions: How to Convert Metric System Prefixes
-Metric unit conversions shortcut: fast, easy how-to with examples
-Mole Conversions Tutorial: how to convert mole - mass, mole - particle, mass - particle problems
-Frequency, Wavelength, and the Speed of Light
-The Bohr Model of the Atom and Atomic Emission Spectra
-What is Heat: A brief introduction at the particle level
-Rutherford's Gold Foil Experiment
-Unit Conversion Using Dimensional Analysis Tutorial
-What is Fire: Combustion Reaction Tutorial
-Quantum Numbers Tutorial
-Electron Configurations Tutorial and How to Derive Electron Configurations from the Periodic Table
-Concentration and Molarity Explained
-Heating Curves Tutorial
-Naming Ionic Compounds
-Limiting Reactant Tutorial
-PV=nRT The Ideal Gas Law: What is it, What is R, Four practice problems solved including molar mass
-Gas density and PV=nRT, the ideal gas law
-Surface Tension - What is it, how does it form, what properties does it impart
---
—More on Atomic Orbitals | Wikipedia—
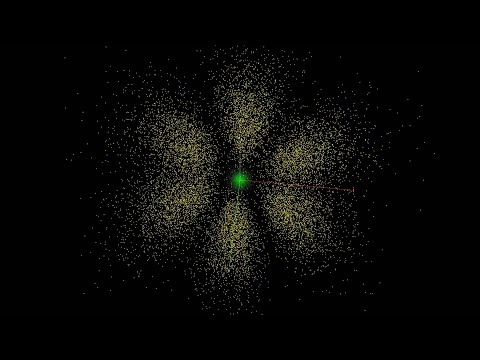
"An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom.[1] This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term, atomic orbital, may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.[2]
Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number s. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. ...
Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively, although for higher values of the quantum number n, particularly when the atom in question bears a positive charge, the energies of certain sub-shells become very similar and so the order in which they are said to be populated by electrons (e.g. Cr = [Ar]4s13d5 and Cr2+ = [Ar]3d4) can only be rationalized somewhat arbitrarily."
Wikipedia contributors. "Atomic orbital." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 17 May. 2016. Web. 27 May. 2016.
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
-Stoichiometry Tutorial, step by step
-Types of Chemical Reactions: How to classify five basic reaction types
-Solution Stoichiometry
-Orbitals the Basics: Atomic Orbitals Tutorial
-Hybrid Orbitals Explained
-Polar Molecules Tutorial: How to determine polarity in a molecule
-Metallic Bonding and Metallic Properties Explained
-Covalent Bonding Tutorial
-Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
-Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
-Metric Unit Prefix Conversions: How to Convert Metric System Prefixes
-Metric unit conversions shortcut: fast, easy how-to with examples
-Mole Conversions Tutorial: how to convert mole - mass, mole - particle, mass - particle problems
-Frequency, Wavelength, and the Speed of Light
-The Bohr Model of the Atom and Atomic Emission Spectra
-What is Heat: A brief introduction at the particle level
-Rutherford's Gold Foil Experiment
-Unit Conversion Using Dimensional Analysis Tutorial
-What is Fire: Combustion Reaction Tutorial
-Quantum Numbers Tutorial
-Electron Configurations Tutorial and How to Derive Electron Configurations from the Periodic Table
-Concentration and Molarity Explained
-Heating Curves Tutorial
-Naming Ionic Compounds
-Limiting Reactant Tutorial
-PV=nRT The Ideal Gas Law: What is it, What is R, Four practice problems solved including molar mass
-Gas density and PV=nRT, the ideal gas law
-Surface Tension - What is it, how does it form, what properties does it impart
---
—More on Atomic Orbitals | Wikipedia—
"An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom.[1] This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term, atomic orbital, may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.[2]
Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number s. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. ...
Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively, although for higher values of the quantum number n, particularly when the atom in question bears a positive charge, the energies of certain sub-shells become very similar and so the order in which they are said to be populated by electrons (e.g. Cr = [Ar]4s13d5 and Cr2+ = [Ar]3d4) can only be rationalized somewhat arbitrarily."
Wikipedia contributors. "Atomic orbital." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 17 May. 2016. Web. 27 May. 2016.
Комментарии
 0:14:28
0:14:28
 0:11:19
0:11:19
 0:10:52
0:10:52
 0:21:34
0:21:34
 0:02:50
0:02:50
 0:08:42
0:08:42
 0:05:35
0:05:35
 0:05:50
0:05:50
 0:10:55
0:10:55
 0:10:53
0:10:53
 0:09:23
0:09:23
 0:13:34
0:13:34
 0:00:33
0:00:33
 0:12:12
0:12:12
 0:10:19
0:10:19
 0:08:39
0:08:39
 0:14:28
0:14:28
 0:06:00
0:06:00
 0:07:54
0:07:54
 0:00:48
0:00:48
 0:06:05
0:06:05
 0:08:11
0:08:11
 0:05:24
0:05:24
 0:00:16
0:00:16