filmov
tv
18.1 The Laws of Thermodynamics | General Chemistry

Показать описание
Chad provides an introduction to Thermodynamics describing the Three Laws of Thermodynamics.
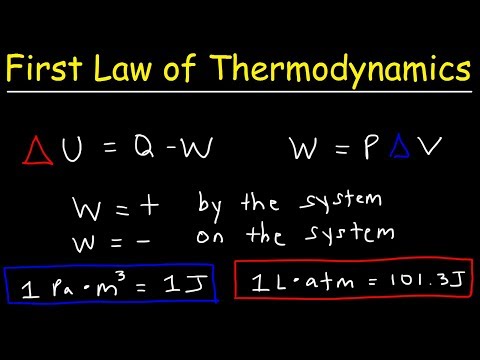
The First Law of Thermodynamics, covered originally in chapter 5, can be summarized as stating that energy cannot be created or destroyed.
The Second Law of Thermodynamics states that for a spontaneous process the entropy of the universe increases.
The Third Law of Thermodynamics states that a perfect crystal at zero Kelvin has zero entropy.
00:00 Lesson Introduction
00:23 1st Law of Thermodynamics
03:23 2nd Law of Thermodynamics
07:37 3rd Law of Thermodynamics
The First Law of Thermodynamics, covered originally in chapter 5, can be summarized as stating that energy cannot be created or destroyed.
The Second Law of Thermodynamics states that for a spontaneous process the entropy of the universe increases.
The Third Law of Thermodynamics states that a perfect crystal at zero Kelvin has zero entropy.
00:00 Lesson Introduction
00:23 1st Law of Thermodynamics
03:23 2nd Law of Thermodynamics
07:37 3rd Law of Thermodynamics
18.1 The Laws of Thermodynamics | General Chemistry
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
First Law of Thermodynamics, Basic Introduction, Physics Problems
First Law of Thermodynamics.
Thermodynamics: Crash Course Physics #23
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
Simplifying the First Law of Thermodynamics | Physics by Parth G
Additional Short Questions | Chapter 11 | Heat and Thermodynamics | Class 11 Physics
The Zeroth Law of Thermodynamics: Thermal Equilibrium
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
Understanding Second Law of Thermodynamics !
What Is 'Entropy?'
Lesson 1: Intro to Thermodynamics
Enthalpy: Crash Course Chemistry #18
Boyle’s Law
First and Second Law of Thermodynamics
First Law of Thermodynamics [year-1]
The 3 Laws of Thermodynamics
Chapter 18 — Thermodynamics
PHY S 100 Chapter 18 | The Law of Increasing Disorder
Lecture 18: Third law of thermodynamics
VCE Physics unit 1: Heat (part 2): The First Law of Thermodynamics
3rd Law of Thermodynamics.
Комментарии
 0:10:06
0:10:06
 0:08:12
0:08:12
 0:11:27
0:11:27
 0:10:31
0:10:31
 0:00:29
0:00:29
 0:10:04
0:10:04
 0:04:11
0:04:11
 0:07:39
0:07:39
 0:08:24
0:08:24
 0:03:29
0:03:29
 0:10:05
0:10:05
 0:06:56
0:06:56
 0:01:00
0:01:00
 0:05:44
0:05:44
 0:11:24
0:11:24
 0:00:15
0:00:15
 0:14:03
0:14:03
 0:08:40
0:08:40
 0:04:09
0:04:09
 0:28:05
0:28:05
 0:04:04
0:04:04
 0:31:49
0:31:49
 0:05:36
0:05:36
 0:00:18
0:00:18