filmov
tv
Ions and Isotopes | Chemistry Animation

Показать описание
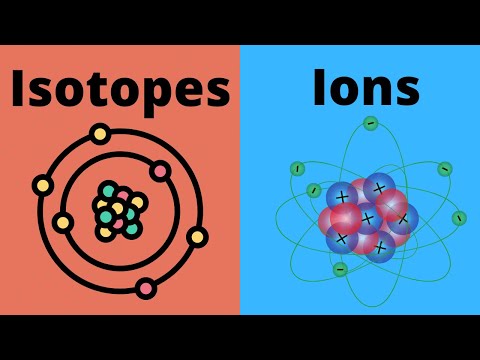
What are Ions and Isotopes?
For our second video in the series discussion about Atoms, we will be understanding the classification of atoms according to their present electrical charges, which are IONS and ISOTOPES.
We have previously learned that atoms are the building block of matter, which is practically everything we see in the universe. Atoms are made up of three subatomic particles, which are the electrons or the negatively charged particle, the proton or the positively charged particle, and the neutron or the neutrally charged particle.
Did you know? That, the difference between atoms and elements is that an element is composed of a distinct atom made up of a specific number of protons. This distinction was the basis to create the Table of Elements.
From that, we basically know that the number of protons in every atom is distinct and constant, but the number of electrons and neutrons may vary. An atom with the same number of electrons and protons is called a neutral atom because, with the same number of opposite electrical charges, it cancels out both of the electrical charges. But as we can say, the number of electrons may vary because an atom can gain or lose an electron. Once an atom carries a certain electrical charge, it is then called an ion. An atom that loses electrons creates itself as a positively charged ion called cation. While if an atom gains an electron, it becomes a negatively charged ion that is called an anion.
Enjoy this video about Ions and Isotopes.
#Ions&Isotopes #Chemistry #EducationalVideo
CONTACT US
For our second video in the series discussion about Atoms, we will be understanding the classification of atoms according to their present electrical charges, which are IONS and ISOTOPES.
We have previously learned that atoms are the building block of matter, which is practically everything we see in the universe. Atoms are made up of three subatomic particles, which are the electrons or the negatively charged particle, the proton or the positively charged particle, and the neutron or the neutrally charged particle.
Did you know? That, the difference between atoms and elements is that an element is composed of a distinct atom made up of a specific number of protons. This distinction was the basis to create the Table of Elements.
From that, we basically know that the number of protons in every atom is distinct and constant, but the number of electrons and neutrons may vary. An atom with the same number of electrons and protons is called a neutral atom because, with the same number of opposite electrical charges, it cancels out both of the electrical charges. But as we can say, the number of electrons may vary because an atom can gain or lose an electron. Once an atom carries a certain electrical charge, it is then called an ion. An atom that loses electrons creates itself as a positively charged ion called cation. While if an atom gains an electron, it becomes a negatively charged ion that is called an anion.
Enjoy this video about Ions and Isotopes.
#Ions&Isotopes #Chemistry #EducationalVideo
CONTACT US
Комментарии
 0:04:22
0:04:22
 0:05:29
0:05:29
 0:05:04
0:05:04
 0:05:33
0:05:33
 0:05:25
0:05:25
 0:04:10
0:04:10
 0:05:22
0:05:22
 0:07:01
0:07:01
 0:02:59
0:02:59
 0:08:43
0:08:43
 0:12:42
0:12:42
 0:03:34
0:03:34
 0:03:31
0:03:31
 0:07:20
0:07:20
 0:02:51
0:02:51
 0:03:19
0:03:19
 0:10:22
0:10:22
 0:04:47
0:04:47
 0:03:44
0:03:44
 0:04:34
0:04:34
 0:01:24
0:01:24
 0:01:55
0:01:55
 0:03:01
0:03:01
 0:10:31
0:10:31