filmov
tv
Isotopes vs Ions | What is the Difference? |
Показать описание
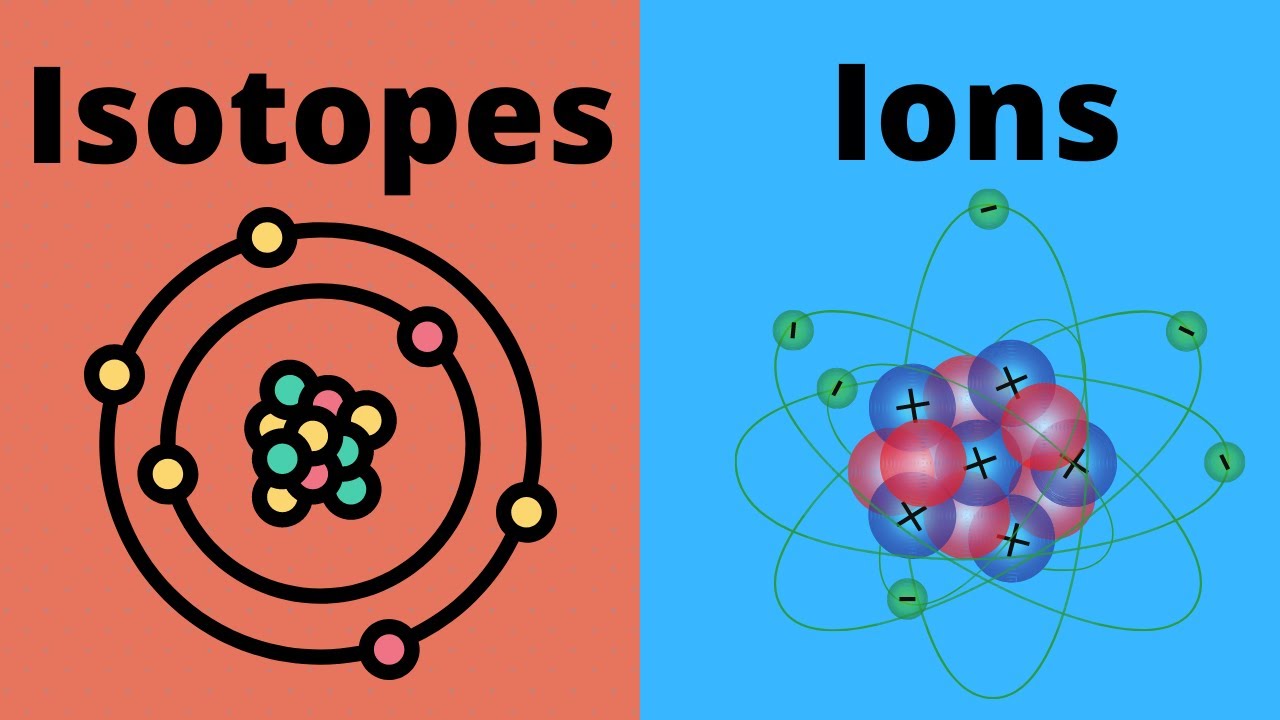
Learn the difference between an isotope and an ion.
An isotope has the same number of protons but a different number of neutrons.
An ion has the same number of protons but a different number of electrons.
For more Math help visit our website
An isotope has the same number of protons but a different number of neutrons.
An ion has the same number of protons but a different number of electrons.
For more Math help visit our website
Isotopes vs Ions | What is the Difference? |
Isotopes vs Ions (The difference between isotopes and ions.)
Nuclide Symbols: Atomic Number, Mass Number, Ions, and Isotopes
Ions and Isotopes | Chemistry Animation
Atoms, Ions, and Isotopes
Isotopes Vs Ions What is the difference??
Isotopes: The Siblings of Atoms
What are Isotopes?
DIFFERENCE BETWEEN ISOTOPE, ISOBAR, ISOTONE AND ISOELECTRONIC- (STRUCTURES OF ATOM)
Key Concept: Isotopes vs Ions
Ions and isotopes
Worked example: Identifying isotopes and ions | Chemistry | Khan Academy
Atoms, Ions, and Isotopes
Isotopes and Ions
Average atomic mass | Atoms, isotopes, and ions | AP Chemistry | Khan Academy
Atoms: Ions and Isotopes
Isotopes | Atoms, isotopes, and ions | High school chemistry | Khan Academy
N5 Chemistry Isotopes and Ions
What is an ion? | Cation vs Anion
Neutral Atoms, Ions, and Isotopes
Ions and Isotopes (with Primrose Kitten) - GCSE Physics
Ions and Isotopes
GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
Elements, Atoms, Molecules, Ions, Ionic and Molecular Compounds, Cations vs Anions, Chemistry
Комментарии
 0:04:22
0:04:22
 0:05:33
0:05:33
 0:05:04
0:05:04
 0:05:29
0:05:29
 0:05:25
0:05:25
 0:13:39
0:13:39
 0:02:59
0:02:59
 0:12:42
0:12:42
 0:09:56
0:09:56
 0:27:59
0:27:59
 0:03:34
0:03:34
 0:03:44
0:03:44
 0:08:43
0:08:43
 0:11:25
0:11:25
 0:08:38
0:08:38
 0:06:55
0:06:55
 0:04:10
0:04:10
 0:18:11
0:18:11
 0:02:30
0:02:30
 0:04:47
0:04:47
 0:03:26
0:03:26
 0:05:46
0:05:46
 0:07:01
0:07:01
 0:13:53
0:13:53