filmov
tv
Isotopes vs Ions (The difference between isotopes and ions.)

Показать описание
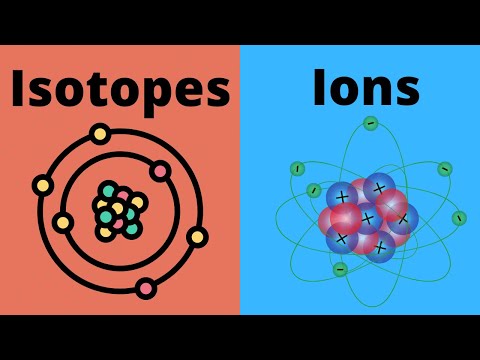
To understand the difference between isotopes and ions we first look to their definitions. Isotopes are versions of a particular element that have different numbers of neutrons. Ions are atoms (or molecules) that have lost or gained electrons and have an electrical charge.
Ions will have a + or – sign after the element symbol.
Na+ , Mg2+ , Cl - , O2-, N3-
Not that there are also polyatomic ions, like SO4 2- , which are a group of atoms bonded together. The resulting compound has an overall charge.
“Can Isotopes also be Ions?”
Atom Builder shown in video:
Many elements on the periodic table more than one isotopes. Tin has ten.
Most atoms can also become ions with varying degrees of ease.
Neutrons are difficult to add or remove. You usually need a super nova explosion or a particle accelerator. Nuclear decay can also result in the formation of isotopes.
One last thing, in general, the isotopes of an element behave mostly the same. We sort of ignore them in chemistry. But ions behave very differently. Na+ in water, no problem. Na in water… boom.
Much of chemistry and chemical reactions involve ions.
Ions will have a + or – sign after the element symbol.
Na+ , Mg2+ , Cl - , O2-, N3-
Not that there are also polyatomic ions, like SO4 2- , which are a group of atoms bonded together. The resulting compound has an overall charge.
“Can Isotopes also be Ions?”
Atom Builder shown in video:
Many elements on the periodic table more than one isotopes. Tin has ten.
Most atoms can also become ions with varying degrees of ease.
Neutrons are difficult to add or remove. You usually need a super nova explosion or a particle accelerator. Nuclear decay can also result in the formation of isotopes.
One last thing, in general, the isotopes of an element behave mostly the same. We sort of ignore them in chemistry. But ions behave very differently. Na+ in water, no problem. Na in water… boom.
Much of chemistry and chemical reactions involve ions.
Комментарии
 0:04:22
0:04:22
 0:05:33
0:05:33
 0:02:37
0:02:37
 0:05:04
0:05:04
 0:05:29
0:05:29
 0:27:59
0:27:59
 0:13:39
0:13:39
 0:05:25
0:05:25
 0:03:34
0:03:34
 0:02:59
0:02:59
 0:12:42
0:12:42
 0:02:54
0:02:54
 0:04:10
0:04:10
 0:01:55
0:01:55
 0:04:06
0:04:06
 0:00:05
0:00:05
 0:02:08
0:02:08
 0:11:25
0:11:25
 0:00:31
0:00:31
 0:02:59
0:02:59
 0:02:06
0:02:06
 0:05:46
0:05:46
 0:09:46
0:09:46
 0:08:26
0:08:26