filmov
tv
Measuring Enthalpy Changes (IB Chemistry R1.1)

Показать описание
IB Chemistry Reactivity 1 - Thermochemistry - Measuring Enthalpy Changes
In this video we will be looking at what calorimetry is and how we can use temperature changes and specific heat capacity to calculate energy changes. We will then go through a few different examples of how to apply our understanding.
Specific heat capacity 1:08
Calorimetry 2:11
Calculating enthalpy changes 3:15
Example calculations 7:09
In this video we will be looking at what calorimetry is and how we can use temperature changes and specific heat capacity to calculate energy changes. We will then go through a few different examples of how to apply our understanding.
Specific heat capacity 1:08
Calorimetry 2:11
Calculating enthalpy changes 3:15
Example calculations 7:09
Enthalpy Changes [IB Chemistry SL/HL]
R1.2.3/5.1 Standard Enthalpy Change of Formation and Combustion [HL IB Chemistry 2024]
IB Chem - Enthalpy change
Enthalpy change calculation IB Chemistry
IB Chemistry Topic 5.1 Measuring energy changes
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19M.2.SL.TZ1.8c
R1.1.4 Enthalpy and enthalpy change
Required practical 2: Measurement of an enthalpy change
5.1 Calculating enthalpy changes (SL)
Calculating enthalpy change
Hess's Law Problems & Enthalpy Change - Chemistry
Measuring Enthalpy Changes (IB Chemistry R1.1)
IB Chemistry: Calculating Enthalpy Changes Experimentally
5.1 Evaluate experiments to determine enthalpy changes [SL IB Chemistry]
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 20N.1A.SL.TZ0.28
5.3/R1.2.1 Average Bond Enthalpy Calculations [SL IB Chemistry]
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 22M.1A.SL.TZ1.13
5.1 Calculate enthalpy change for a reaction using experimental data (mcdeltaT) [SL IB Chemistry]
Enthalpy Changes Practical As Level Chemistry
The EASIEST Method For Solving Hess Cycles
IB Chemistry Energetics Revision Workshop HL/SL (Topic 5/15)
Hess' Law (Enthalpy Changes) - IB Chemistry Revision Course
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19N.1A.SL.TZ0.13
IB Chemistry - How to calculate enthalpy change using formation enthalpies (Topic 5)
Комментарии
 0:11:56
0:11:56
 0:08:00
0:08:00
 0:06:06
0:06:06
 0:05:18
0:05:18
 0:41:56
0:41:56
 0:05:11
0:05:11
 0:01:29
0:01:29
 0:07:09
0:07:09
 0:08:01
0:08:01
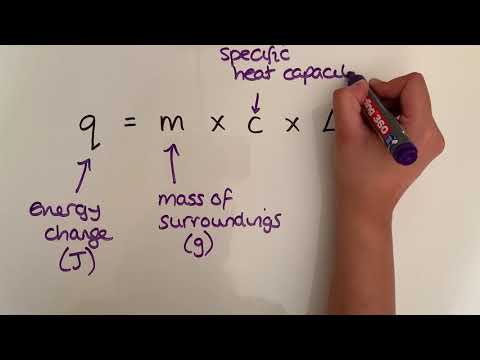 0:07:22
0:07:22
 0:14:03
0:14:03
 0:18:35
0:18:35
 0:05:35
0:05:35
 0:01:45
0:01:45
 0:04:28
0:04:28
 0:06:04
0:06:04
 0:04:59
0:04:59
 0:09:30
0:09:30
 0:04:05
0:04:05
 0:13:46
0:13:46
 1:12:16
1:12:16
 0:12:07
0:12:07
 0:05:01
0:05:01
 0:05:26
0:05:26