filmov
tv
5.1 Calculate enthalpy change for a reaction using experimental data (mcdeltaT) [SL IB Chemistry]

Показать описание
5.2.3 Calculate the enthalpy change for a reaction using experimental data on temperature changes, quantities of reactants and mass of water. Burning a known mass of fuel and causing a known mass of water to be heated by a certain amount will allow you to calculate delta H using
delta H = -m c deltaT
Where m = mass of what is heated
c=specific heat capacity of what is heated
delta T = temperature change of what is heated.
Dr Atkinson has been warned to never EVER play around near calorimeters again!
delta H = -m c deltaT
Where m = mass of what is heated
c=specific heat capacity of what is heated
delta T = temperature change of what is heated.
Dr Atkinson has been warned to never EVER play around near calorimeters again!
5.1 Calculating enthalpy changes (SL)
Hess's Law Problems & Enthalpy Change - Chemistry
Calculating enthalpy change
5.1 Calculate enthalpy change for a reaction using experimental data (mcdeltaT) [SL IB Chemistry]
[Example] How to Calculate Enthalpy Change of a Reaction.
Enthalpy Changes [IB Chemistry SL/HL]
CHEM 101 - Using Hess's Law to Calculate Enthalpy Change
Energy & Chemical Change| L4: Calculating Enthalpy Change 'Part 1' @EasyChemistry4all
IB Chemistry - How to calculate enthalpy change using formation enthalpies (Topic 5)
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
5.2.1 - 5.2.4 Calculating enthalpy changes from experimental data
R1.1.4 Calculating ΔH using q = mcΔT
Required practical 2: Measurement of an enthalpy change
GCSE Chemistry - Bond Energies #44 (Higher tier)
Enthalpies of solution
Bond Enthalpies (Bond Energies) to Calculate Enthalpy Change - Thermodynamics
5 Equations to calculate the heat or enthalpy change of physical processes
CHEM 101 - Calculating Enthalpy of Solution
A-level chemistry - experimental method to calculate the enthalpy change of reaction, exam question
Calculate Enthalpy, Enthropy Change for Reaction
Enthalpy changes question (AQA A Level Chemistry)
IB Chemistry Topic 5 Energetics 5.2 Hess's Law with enthalpy of formation and enthalpy of combu...
Enthalpy Stoichiometry Part 1: Finding Heat and Mass
Hess's Law Example Problem
Комментарии
 0:08:01
0:08:01
 0:14:03
0:14:03
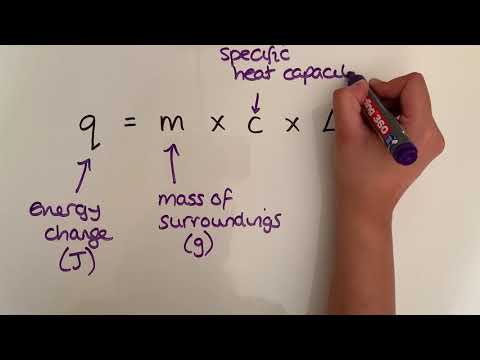 0:07:22
0:07:22
 0:09:30
0:09:30
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
 0:11:56
0:11:56
 0:04:45
0:04:45
 0:27:55
0:27:55
 0:05:26
0:05:26
 0:16:42
0:16:42
 0:06:15
0:06:15
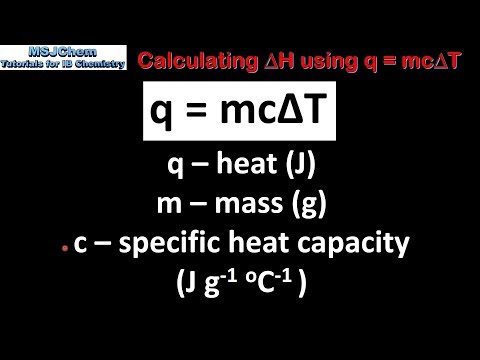 0:03:35
0:03:35
 0:07:09
0:07:09
 0:04:23
0:04:23
 0:07:38
0:07:38
 0:04:24
0:04:24
 0:06:46
0:06:46
 0:02:56
0:02:56
 0:05:17
0:05:17
 0:07:17
0:07:17
 0:18:23
0:18:23
 0:07:40
0:07:40
 0:05:50
0:05:50
 0:04:11
0:04:11