filmov
tv
Calculating enthalpy change

Показать описание
Calculating enthalpy change
Hess's Law Problems & Enthalpy Change - Chemistry
[Example] How to Calculate Enthalpy Change of a Reaction.
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
5.1 Calculating enthalpy changes (SL)
Hess's Law and Heats of Formation
Enthalpy Changes [IB Chemistry SL/HL]
The EASIEST Method For Solving Hess Cycles
Enthalpy: Crash Course Chemistry #18
CHEM 101 - Using Hess's Law to Calculate Enthalpy Change
GCSE Chemistry - Bond Energies #44 (Higher tier)
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Tutorial: Calculating Molar Enthalpy Change
Required practical 2: Measurement of an enthalpy change
Thermochemistry: Heat and Enthalpy
Inspire Chemistry| Module 14| Lesson 4: Calculating Enthalpy Change 'Part 1' @EasyChemistr...
Enthalpy Calculations
Calculating enthalpy change of combustion
Enthalpy of reaction | Thermodynamics | AP Chemistry | Khan Academy
Hess's Law Common Test Question
Enthalpy | Thermodynamics
Enthalpy Stoichiometry Part 1: Finding Heat and Mass
Enthalpies of Reactions - Using Average Bond Enthalpies - Chemistry Tutorial
R1.1.4 Calculating ΔH using q = mcΔT
Комментарии
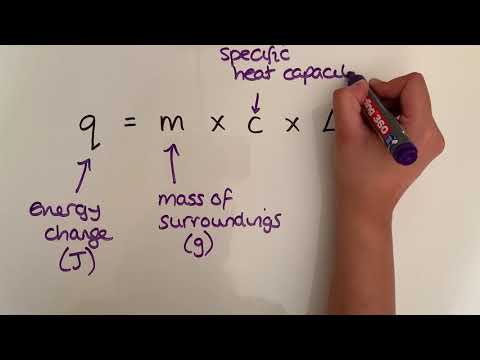 0:07:22
0:07:22
 0:14:03
0:14:03
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
 0:11:39
0:11:39
 0:08:01
0:08:01
 0:04:58
0:04:58
 0:11:56
0:11:56
 0:13:46
0:13:46
 0:11:24
0:11:24
 0:04:45
0:04:45
 0:04:23
0:04:23
 0:16:42
0:16:42
 0:03:31
0:03:31
 0:07:09
0:07:09
 0:04:17
0:04:17
 0:27:55
0:27:55
 0:05:36
0:05:36
 0:15:26
0:15:26
 0:04:17
0:04:17
 0:03:11
0:03:11
 0:10:55
0:10:55
 0:05:50
0:05:50
 0:07:49
0:07:49
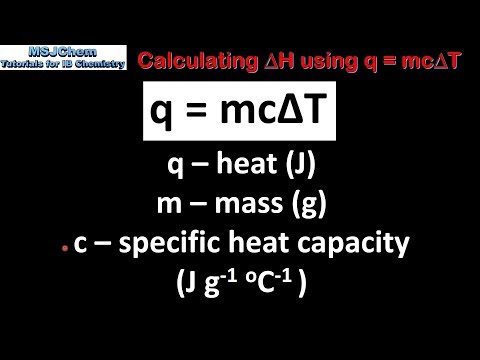 0:03:35
0:03:35