filmov
tv
R1.2.1 Average Bond Enthalpy Calculations [SL IB Chemistry]
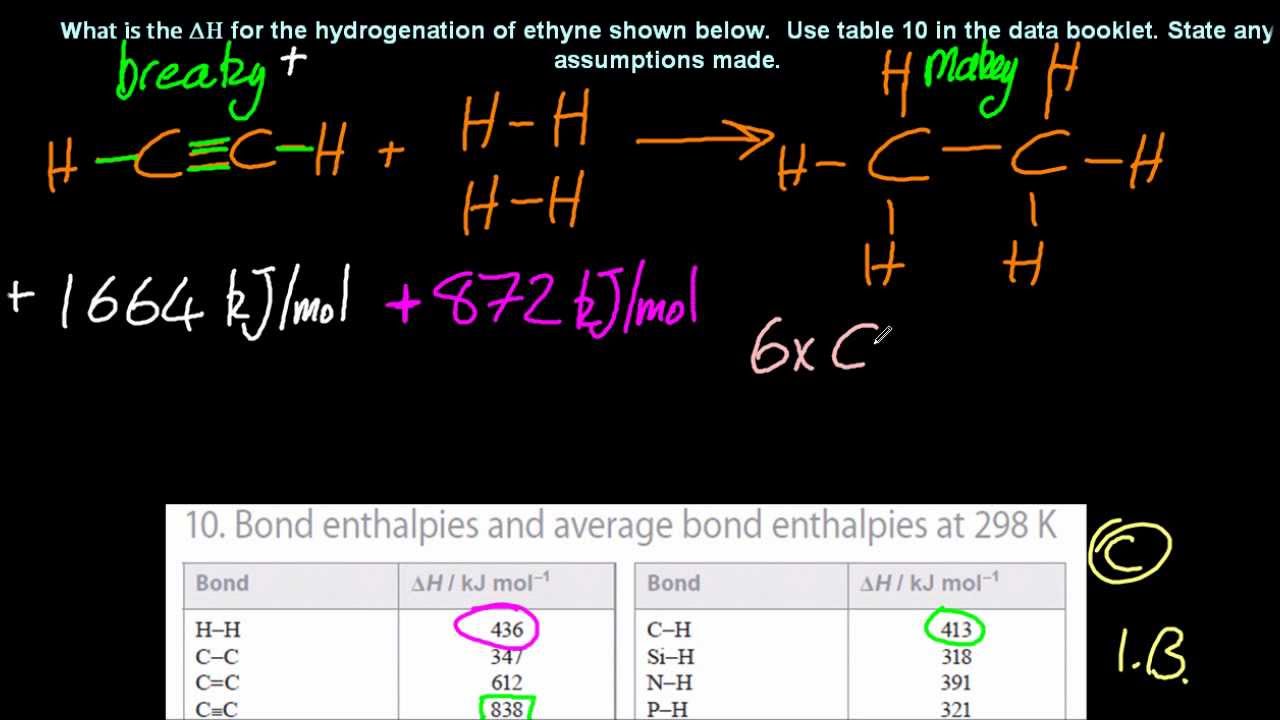
Показать описание
If you know the energy required to break all the bonds in the reactants AND make all the bonds in the products you can work out the overall energy change for the reaction. Assume STP and this only works if everything involved is a gas. (Else the energy you put in may cause evaporation, boiling, hydrogen-bond breaking etc).
R1.2.1 Average Bond Enthalpy Calculations [SL IB Chemistry]
R1.2.1 Calculating ΔH using average bond enthalpies
R1.2.1 - How do we apply Hess's law to average bond enthalpies?
Bond Enthalpy Calculations (IB Chemistry R1.2)
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
GCSE Chemistry - Bond Energies #44 (Higher tier)
5.3 Bond enthalpies - calculate enthalpy of reaction using average bond enthalpy
3 Average Bond Enthalpies
5.4.1 - 5.4.2 Average bond enthalpies
Determine Enthalpy Change Using Average Bond Energy
Enthalpy changes 3 average bond enthalpy
enthalpy calculations using average bond enthalpies part 2
CHEMISTRY 101 - Average bond energies to calculate change in enthalpy
Calculating bond enthalpy from average bond energies
Calculate Change in Enthalpy (using Bond Energy)
Using average bond enthalpies
Using average bond enthalpies
Worked example: Using bond enthalpies to calculate enthalpy of reaction | Khan Academy
Bond Energy Calculations
PRQ Average bond energies
5.3 Bond enthalpies (SL)
Standard, Average Bond Enthalpies
Bond Enthalpies (AQA A level Chemistry)
Bond Enthalpy Calculations
Комментарии
 0:06:04
0:06:04
 0:05:16
0:05:16
 0:06:40
0:06:40
 0:10:18
0:10:18
 0:11:39
0:11:39
 0:04:23
0:04:23
 0:03:43
0:03:43
 0:10:23
0:10:23
 0:04:08
0:04:08
 0:12:08
0:12:08
 0:07:26
0:07:26
 0:07:51
0:07:51
 0:01:56
0:01:56
 0:10:19
0:10:19
 0:04:45
0:04:45
 0:09:18
0:09:18
 0:09:17
0:09:17
 0:09:03
0:09:03
 0:12:24
0:12:24
 0:00:26
0:00:26
 0:03:42
0:03:42
 0:04:23
0:04:23
 0:19:46
0:19:46
 0:13:49
0:13:49