filmov
tv
5.1 Calculating enthalpy changes (SL)

Показать описание
This video covers how to calculate enthalpy changes in
neutralization and combustion reactions.
Link to practice worksheet:
neutralization and combustion reactions.
Link to practice worksheet:
5.1 Calculating enthalpy changes (SL)
IB Chemistry Topic 5.1: Measuring Energy Changes
Enthalpy Changes [IB Chemistry SL/HL]
Discussion 5-1 Experimental and Average Bond Enthalpy Calculations HL/SL
5.1 Evaluate experiments to determine enthalpy changes [SL IB Chemistry]
Topic 5 Enthalpy Change Questions IB SL and HL
Calculating enthalpy change
IB Chemistry Topic 5 Energetics 5.1 Q=mcdt - heat and calculations
Hess's Law Problems & Enthalpy Change - Chemistry
IB Chemistry - How to calculate enthalpy change using formation enthalpies (Topic 5)
Enthalpy change calculation IB Chemistry
5.1 Calculate enthalpy change for a reaction using experimental data (mcdeltaT) [SL IB Chemistry]
IB Chemistry Energetics Revision Workshop HL/SL (Topic 5/15)
The EASIEST Method For Solving Hess Cycles
R1.1.4 Calculating ΔH using q = mcΔT
R1.1.4 Enthalpy and enthalpy change
5.1 Enthalpy changes (SL)
IB Chemistry SL | Reactivity 1.1 - Measuring Enthalpy Changes | 19M.2.SL.TZ1.8c
[Example] How to Calculate Enthalpy Change of a Reaction.
IB Chemistry Topic 5 Energetics 5.1 Measuring energy changes with Q=mcdT
Hess's Law and Heats of Formation
R1.2.3/5.1 Standard Enthalpy Change of Formation and Combustion [HL IB Chemistry 2024]
Required practical 2: Measurement of an enthalpy change
GCSE Chemistry - Bond Energies #44 (Higher tier)
Комментарии
 0:08:01
0:08:01
 0:05:52
0:05:52
 0:11:56
0:11:56
 0:23:25
0:23:25
 0:01:45
0:01:45
 0:10:40
0:10:40
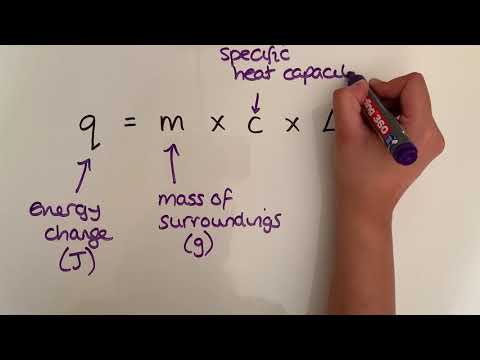 0:07:22
0:07:22
 0:06:21
0:06:21
 0:14:03
0:14:03
 0:05:26
0:05:26
 0:05:18
0:05:18
 0:09:30
0:09:30
 1:12:16
1:12:16
 0:13:46
0:13:46
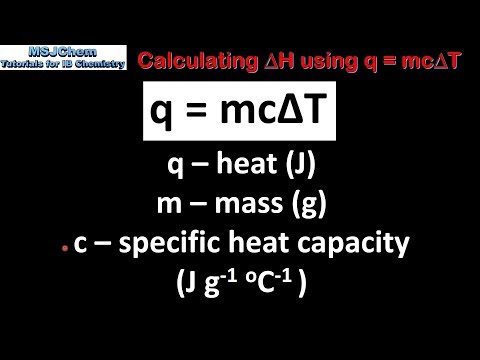 0:03:35
0:03:35
 0:01:29
0:01:29
 0:02:16
0:02:16
 0:05:11
0:05:11
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
 0:11:54
0:11:54
 0:04:58
0:04:58
 0:08:00
0:08:00
 0:07:09
0:07:09
 0:04:23
0:04:23