filmov
tv
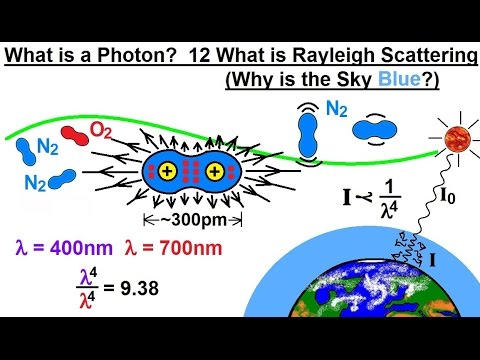
Particle Physics (28 of 41) What is a Photon? 12. Rayleigh Scattering (Why is the Sky Blue?)

Показать описание
In this video I will explain Rayleigh scattering and why is the sky blue?
Next video in the Particle Physics series can be seen at:
Particle Physics (28 of 41) What is a Photon? 12. Rayleigh Scattering (Why is the Sky Blue?)
Particle Physics (34 of 41) What is a Photon? 18. Amplitude vs Intensity - How 'Big' is a ...
Particle Physics (26 of 41) What is a Photon? 10. Momentum and Velocity
Particle Physics (29 of 41) What is a Photon? 13. Mie Scattering
Particle Physics (33 of 41) What is a Photon? 17. Mie Scattering - Radio Waves vs Sunlight
Particle Physics (27 of 41) What is a Photon? 11. Volume (Density) of Photons
Particle Physics (41 of 41) What is a Photon? 25. Atmospheric Water Vapor Absorption
Particle Physics (31 of 41) What is a Photon? 15. Mie Scattering - Radar Cross Section
LIVE | PLUS ONE PHYSICS | SYSTEM OF PARTICLES AND ROTATION MOTION | AEGON #plusonephysics
Particle Physics (1 of 41) The Atom: 'What Is It?'
Particle Physics (32 of 41) What is a Photon? 16. Mie Scattering - Radio Waves Quantized as Photons?
Particle Physics (40 of 41) What is a Photon? 24. Atmospheric CO2 Atmosphere
Particle Physics (10 of 41) Subatomic Particle (Leptons, Quarks, Higgs, Gravitons) 1
Particle Physics (30 of 41) What is a Photon? 14. Mie Scattering (Continued 2)
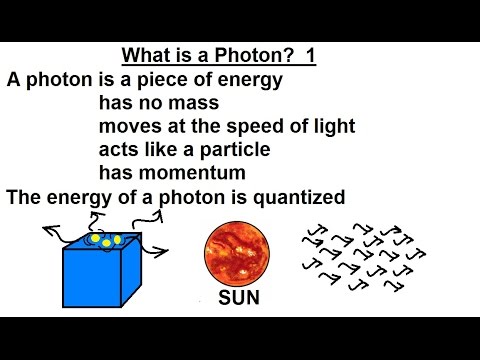
Particle Physics (17 of 41) What is a Photon?
Particle Physics (18 of 41) What is a Photon? 2. Quantum jumps in an Atom
Particle Physics (38 of 41) What is a Photon? 22. UV Rays - How Do We Get Sunburns?
Particle Physics (24 of 41) What is a Photon? 8. How Are X-Rays Produced?
Particle Physics (25 of 41) What is a Photon? 9. Compton Scattering
Particle Physics (2 of 41) The Structure of Atoms
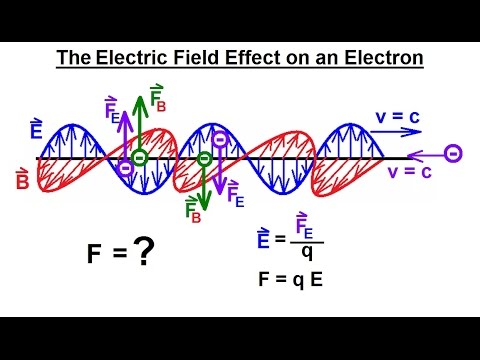
Particle Physics (36 of 41) What is a Photon? 20. The Electric Field
Particle Physics (16 of 41) Elementary Particles: How Are Mesons Made From Quarks?
Particle Physics (9 of 41) The Gravitational Force
Particle Physics (3 of 41) Quantum Mechanics and Special Relativity
Комментарии
 0:09:29
0:09:29
 0:09:47
0:09:47
 0:14:16
0:14:16
 0:08:18
0:08:18
 0:10:19
0:10:19
 0:09:58
0:09:58
 0:03:31
0:03:31
 0:07:29
0:07:29
 0:56:01
0:56:01
 0:05:28
0:05:28
 0:12:01
0:12:01
 0:09:08
0:09:08
 0:08:47
0:08:47
 0:03:27
0:03:27
 0:13:15
0:13:15
 0:08:12
0:08:12
 0:07:45
0:07:45
 0:07:47
0:07:47
 0:12:52
0:12:52
 0:06:53
0:06:53
 0:06:44
0:06:44
 0:04:03
0:04:03
 0:08:02
0:08:02
 0:06:08
0:06:08