filmov
tv
Thermodynamics: 1st Law for Closed Systems (8 of 25)

Показать описание
0:00:13 - Reminders of how to find properties and first law for closed systems
0:02:38 - Example: First law for closed system, rigid tank
0:33:13 - Example: First law for closed system, rigid tank
1:02:37 - Example: First law for closed system, piston-cylinder (continued next lecture)
This lecture series was recorded live at Cal Poly Pomona during Winter 2017. The textbook is Cengel and Boles, "Thermodynamics: An Engineering Approach (8th edition)."
0:02:38 - Example: First law for closed system, rigid tank
0:33:13 - Example: First law for closed system, rigid tank
1:02:37 - Example: First law for closed system, piston-cylinder (continued next lecture)
This lecture series was recorded live at Cal Poly Pomona during Winter 2017. The textbook is Cengel and Boles, "Thermodynamics: An Engineering Approach (8th edition)."
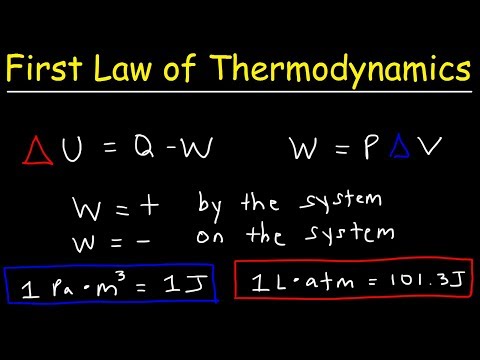
The First Law of Thermodynamics: Internal Energy, Heat, and Work
The first law of Thermodynamics for closed systems | Mechanical Engineering Thermodynamics
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Introduction to First Law: Closed System
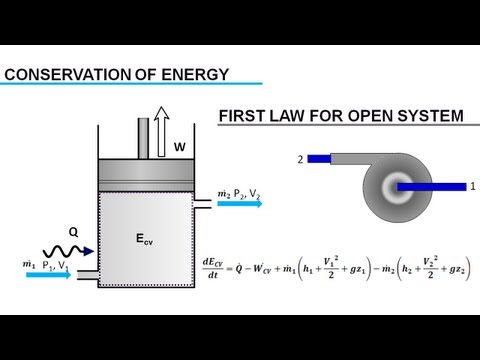
Open System, Closed System and Isolated System - Thermodynamics & Physics
What is the First Law of Thermodynamics?
First Law Of Thermodynamics For A Closed System Undergoing A Change Of State
Thermodynamics: Crash Course Physics #23
ThermoDynamics 🌡️ One Shot | PcmbGeek | Revision Under 15 Minutes #chemistry #thermodynamics
FIRST LAW OF THERMODYNAMICS | Easy and Short
The First & Zeroth Laws of Thermodynamics: Crash Course Engineering #9
First Law of Thermodynamics, Basic Introduction, Physics Problems
The First Law of Thermodynamics | Thermodynamics | (Solved Examples)
Thermodynamics: 1st Law for Closed Systems (8 of 25)
Energy Balance in Closed Systems | Thermodynamics | (Solved examples)
First Law, Second Law, Third Law, Zeroth Law of Thermodynamics
Thermodynamics - Chapter 4 Energy Analysis of Closed Systems
Thermodynamics: 1st Law for Closed Systems, Ideal Gases (10 of 25)
Heat Transfer - First Law of Thermodynamics for Closed System - Thermodynamics
First law of thermodynamics class 11 nbf | 11th class physics ch 10 | kpk, federal, punjab board
First Law of Thermodynamics.
First Law of Thermodynamics
What is 1st law of thermodynamics #thermodynamics #1stlawofthermodynamics #heat
First law of Thermodynamics (Animation)
Комментарии
 0:05:44
0:05:44
 0:07:43
0:07:43
 0:11:27
0:11:27
 0:05:55
0:05:55
 0:03:07
0:03:07
 0:04:09
0:04:09
 0:11:58
0:11:58
 0:10:04
0:10:04
 0:14:02
0:14:02
 0:02:09
0:02:09
 0:10:05
0:10:05
 0:10:31
0:10:31
 0:09:52
0:09:52
 1:06:16
1:06:16
 0:10:43
0:10:43
 0:01:53
0:01:53
 0:09:49
0:09:49
 1:07:43
1:07:43
 0:12:10
0:12:10
 0:26:30
0:26:30
 0:00:29
0:00:29
 0:06:34
0:06:34
 0:00:33
0:00:33
 0:07:01
0:07:01