filmov
tv
Using Gibbs Free Energy

Показать описание
059 - Using Gibbs Free Energy
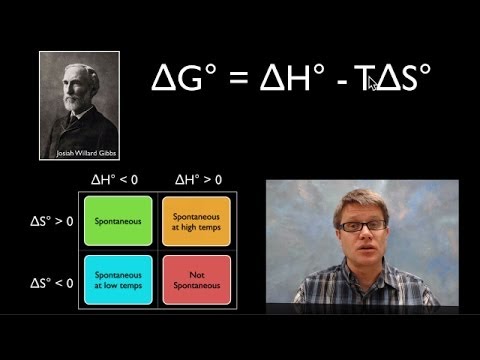
In this video Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. If the ΔG is less than zero the process is spontaneous. If the ΔG is greater than zero the process is not spontaneous. If the ΔG is equal to zero the process is at equilibrium. The ΔH, ΔS, and T are all used to calculate ΔG.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
In this video Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. If the ΔG is less than zero the process is spontaneous. If the ΔG is greater than zero the process is not spontaneous. If the ΔG is equal to zero the process is at equilibrium. The ΔH, ΔS, and T are all used to calculate ΔG.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
Using Gibbs Free Energy
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Gibbs Free Energy
16. Thermodynamics: Gibbs Free Energy and Entropy
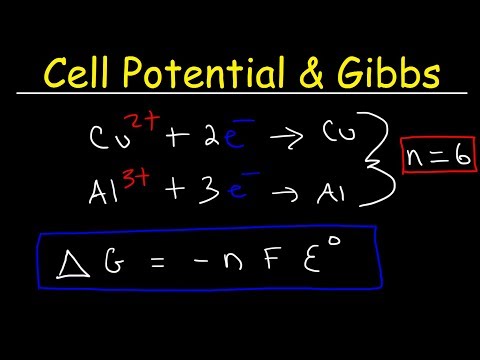
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
Entropy, Enthalpy, & Gibbs Free Energy - Chemistry Spontaneity Formulas
Gibbs free energy and spontaneous reactions | Biology | Khan Academy
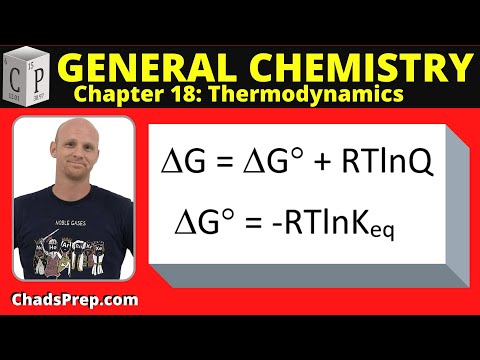
18.5 Gibbs Free Energy and the Equilibrium Constant
Gibbs Free Energy
Gibbs Free Energy (A-Level Chemistry)
Free energy and equilibrium | Applications of thermodynamics | AP Chemistry | Khan Academy
Gibbs Free Energy
Gibbs Free Energy: Concept
Gibbs Free Energy
Using Gibbs free energy to predict reactions
GIbbs Free Energy and Spontaneity
Gibbs Free Energy
Helmholtz and Gibbs Free Energy | Physical Chemistry I | 046
18.5 Gibbs Free Energy and the Equilibrium Constant | General Chemistry
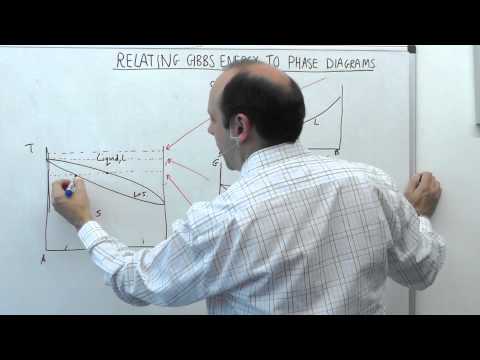
5.2 | MSE104 - Gibbs Energy Curves
Gibbs Free Energy
How to Calculate Change in Gibb's Free Energy of Reaction using Gibbs Formation Example & P...
Комментарии
 0:07:57
0:07:57
 0:08:12
0:08:12
 0:05:39
0:05:39
 0:44:45
0:44:45
 0:14:13
0:14:13
 0:32:01
0:32:01
 0:11:02
0:11:02
 0:11:11
0:11:11
 0:09:17
0:09:17
 0:11:12
0:11:12
 0:05:32
0:05:32
 0:17:41
0:17:41
 0:08:41
0:08:41
 0:10:43
0:10:43
 0:06:14
0:06:14
 0:07:46
0:07:46
 0:49:07
0:49:07
 0:10:52
0:10:52
 0:05:20
0:05:20
 0:13:55
0:13:55
 0:24:43
0:24:43
 0:26:14
0:26:14
 0:03:22
0:03:22
 0:03:29
0:03:29