filmov
tv
Gibbs Free Energy (A-Level Chemistry)

Показать описание
Outlining Gibbs Free Energy and how to calculate it. Feasible and spontaneous reactions are explained. How to find the minimum temperature at which a reaction becomes feasible is explained.
Bit of a marathon - grab a cup of tea!
Recap: 00:37
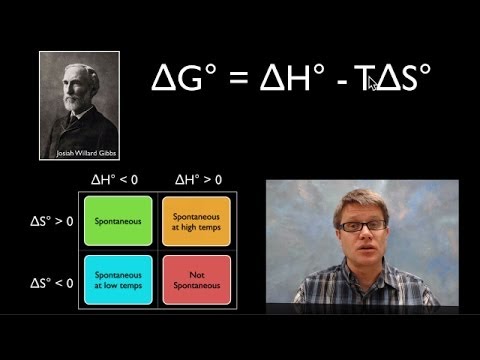
Gibbs Free Energy: 04:20
Feasible reactions: 07:09
‘Possibilities’ for feasible reactions: 10:26
Finding minimum temperature for a reaction to be feasible: 12:31
Summary: 14:18
Relevant Videos:
Bit of a marathon - grab a cup of tea!
Recap: 00:37
Gibbs Free Energy: 04:20
Feasible reactions: 07:09
‘Possibilities’ for feasible reactions: 10:26
Finding minimum temperature for a reaction to be feasible: 12:31
Summary: 14:18
Relevant Videos:
Gibbs Free Energy (A-Level Chemistry)
Gibbs Free Energy | A-Level Chemistry | AQA, OCR, Edexcel
Gibbs Free Energy | A-Level Chemistry
Gibb's Free Energy | Entropy |Thermodynamics | Exam Question Walkthrough | A level Chemistry
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Thermodynamics | Part 2 of 2 | Gibbs Free-Energy Change and Entropy Change | A level Chemistry
Gibbs Free Energy Calculations | Chemistry A-Level
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Gibbs Free Energy Graph - Past Paper Exam Question Walkthrough|A Level Chemistry (AQA)
Gibbs Free Energy | A Level Chemistry | Dr James Vile
A level Chemistry “Entropy and Gibbs Free Energy” Topical Past Paper
Using Gibbs Free Energy
Gibbs Free energy change 1
Entropy, Enthalpy, & Gibbs Free Energy - Chemistry Spontaneity Formulas
Gibbs Free Energy ΔG | A-Level Chemistry | Tailored Tutors
Quick revision - Entropy and Gibbs Free Energy
Thermodynamics: Entropy and Gibbs Free Energy - Exam Question|A-Level Chemistry (AQA)
Gibbs Free Energy, Paper 1 - AQA A Level Chemistry
Entropy: Embrace the Chaos! Crash Course Chemistry #20
Gibbs Free Energy (A2 Chemistry)
Entropy & Gibbs Free Energy | A LEVEL & IB CHEMISTRY | SwH Learning
OCR A 5.2.2 Enthalpy and Entropy REVISION
Thermodynamics: Gibbs Free Energy Calculation - Exam Question|A Level Chemistry (AQA)
R1.4.4 / R2.3.7 Equilibrium and Gibbs free energy (HL)
Комментарии
 0:17:41
0:17:41
 0:03:46
0:03:46
 0:10:51
0:10:51
 0:08:44
0:08:44
 0:08:12
0:08:12
 0:36:34
0:36:34
 0:14:18
0:14:18
 0:44:45
0:44:45
 0:09:41
0:09:41
 0:18:59
0:18:59
 0:10:49
0:10:49
 0:07:57
0:07:57
 0:11:19
0:11:19
 0:11:11
0:11:11
 0:10:10
0:10:10
 0:06:43
0:06:43
 0:07:53
0:07:53
 0:04:09
0:04:09
 0:13:41
0:13:41
 0:17:35
0:17:35
 0:17:38
0:17:38
 0:31:38
0:31:38
 0:08:56
0:08:56
 0:07:07
0:07:07