filmov
tv
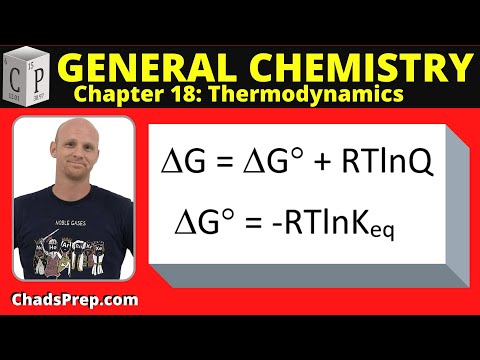
18.5 Gibbs Free Energy and the Equilibrium Constant

Показать описание
Chad explains the relationship between the standard Gibbs Free Energy and the Equilibrium Constant and to perform related calculations.
00:20 - Standard Conditions
02:55 - Delta G under Non-Standard Conditions
04:05 - Delta G Standard (generalizations)
Happy Studying!
00:20 - Standard Conditions
02:55 - Delta G under Non-Standard Conditions
04:05 - Delta G Standard (generalizations)
Happy Studying!
18.5 Gibbs Free Energy and the Equilibrium Constant
Gibbs Free Energy
16. Thermodynamics: Gibbs Free Energy and Entropy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Gibbs free energy - english - lec 5 - Biophysics
Chapter 18. Gibbs Free Energy and Spontaneity
18 2 Example Problems using the Gibbs Free Energy Relationship
Chapter 18. Standard Free Energy of Formation
Thermo 18: Temperature Dependence of Gibbs Energy
Gibbs Free Energy: Chapter 19 – Part 3
Chapter 18: Mathematical relationships between the Gibbs Energy, K and Q.
CH 18 Free Energy & Thermodynamics Part 1
Thermodynamics and Chemical Dynamics 131C. Lecture 18. Equilibrium In Action.
Thermodynamics 5: Gibbs Free Energy and Spontaneity
Gibbs Free Energy Equation and Spontaneity in Chemical Reactions (Exam Problems)
Unit 5 - More With Gibbs Free Energy
Chapter 18: LOTS of Problems with Gibbs Energy, Enthalpy, and Entropy
Thermodynamics Gibbs free energy and Q
What Is Gibbs Free Energy ΔG | What is Thermodynamically Favorable | Calculate Delta G AP Chemistry
AP Chemistry Thermodynamics - Lesson 5 - Gibbs Free Energy, exergonic, endergonic, High/Low temp
Thermodynamics: Gibbs energy and pressure, color-coded derivation
Thermodynamics and Chemical Dynamics 131C. Lecture 15. Getting to Know The Gibbs Energy.
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Calculate Free Energy G from Free Energies of Formation
Комментарии
 0:11:12
0:11:12
 0:14:13
0:14:13
 0:32:01
0:32:01
 0:44:45
0:44:45
 0:10:47
0:10:47
 0:13:00
0:13:00
 0:14:55
0:14:55
 0:07:40
0:07:40
 0:11:53
0:11:53
 0:06:14
0:06:14
 0:09:34
0:09:34
 0:55:32
0:55:32
 0:48:13
0:48:13
 0:05:32
0:05:32
 0:08:51
0:08:51
 0:06:55
0:06:55
 0:29:06
0:29:06
 0:05:15
0:05:15
 0:28:29
0:28:29
 0:24:22
0:24:22
 0:03:44
0:03:44
 0:43:43
0:43:43
 0:05:39
0:05:39
 0:10:53
0:10:53