filmov
tv
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
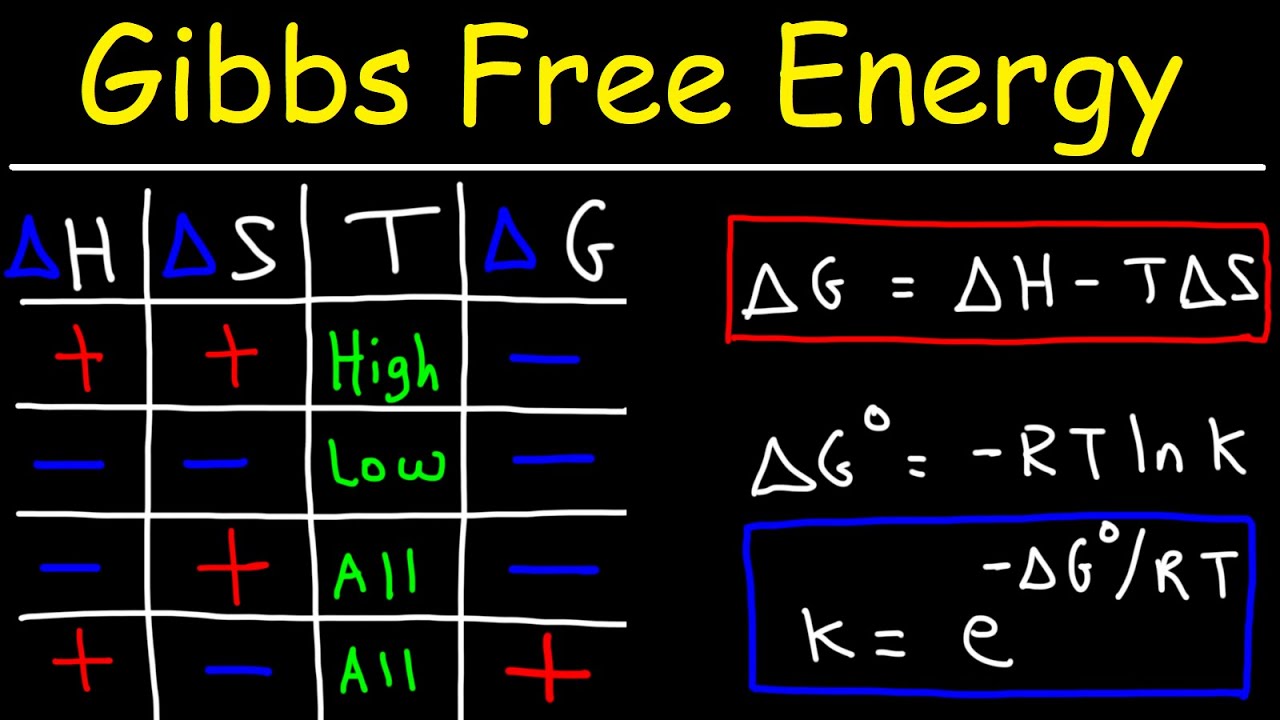
Показать описание
This video provides a basic introduction into Gibbs Free Energy, Entropy, and Enthalpy. It explains how to calculate the equilibrium constant K given the standard free energy change of a reaction and so much more.
Get The Full 1 Hour 49 Min Video:
Direct Link to The Full Video:
PDF Worksheet - 20 Questions:
Chemistry PDF Worksheets:
____________________________
Full 1 Hour 49 Minute Video:
Join The YouTube Membership Program:
General Chemistry 2 Final Exam Review:
Get The Full 1 Hour 49 Min Video:
Direct Link to The Full Video:
PDF Worksheet - 20 Questions:
Chemistry PDF Worksheets:
____________________________
Full 1 Hour 49 Minute Video:
Join The YouTube Membership Program:
General Chemistry 2 Final Exam Review:
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
16. Thermodynamics: Gibbs Free Energy and Entropy
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Using Gibbs Free Energy
Gibbs Free Energy, Entropy, and Enthalpy
Entropy: Embrace the Chaos! Crash Course Chemistry #20
Gibbs Free Energy, Entropy, Thermochemistry Question, Percent Composition, Bohr's Atomic Model
Thermodynamics for Beginners-(For High School Levels Class XI/XII/Competitive Tests)Lecture-5
Quick revision - Entropy and Gibbs Free Energy
What is entropy? - Jeff Phillips
Gibbs Free Energy
Gibbs' Free Energy, Entropy and Enthalpy | Biochemistry
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemis...
Lecture 03, concept 10: Helmholtz vs. Gibbs free energy
Gibbs Free Energy (A-Level Chemistry)
IB Chemistry Topic 15.2 (HL): Entropy & Spontaneity
Gibbs Free energy change 1
Gibbs free energy and spontaneous reactions | Biology | Khan Academy
Gibbs Free Energy Problems
Gibbs Free Energy
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
Second Law of Thermodynamics,Entropy &Gibbs Free Energy
Gibb's Free Energy | Entropy |Thermodynamics | Exam Question Walkthrough | A level Chemistry
Комментарии
 0:08:12
0:08:12
 0:44:45
0:44:45
 0:32:01
0:32:01
 0:05:39
0:05:39
 0:07:57
0:07:57
 0:05:33
0:05:33
 0:13:41
0:13:41
 0:48:43
0:48:43
 3:45:15
3:45:15
 0:06:43
0:06:43
 0:05:20
0:05:20
 0:14:13
0:14:13
 0:16:28
0:16:28
 0:32:10
0:32:10
 0:05:22
0:05:22
 0:17:41
0:17:41
 0:04:59
0:04:59
 0:11:19
0:11:19
 0:09:17
0:09:17
 0:20:26
0:20:26
 0:10:43
0:10:43
 0:04:11
0:04:11
 0:13:50
0:13:50
 0:08:44
0:08:44