filmov
tv
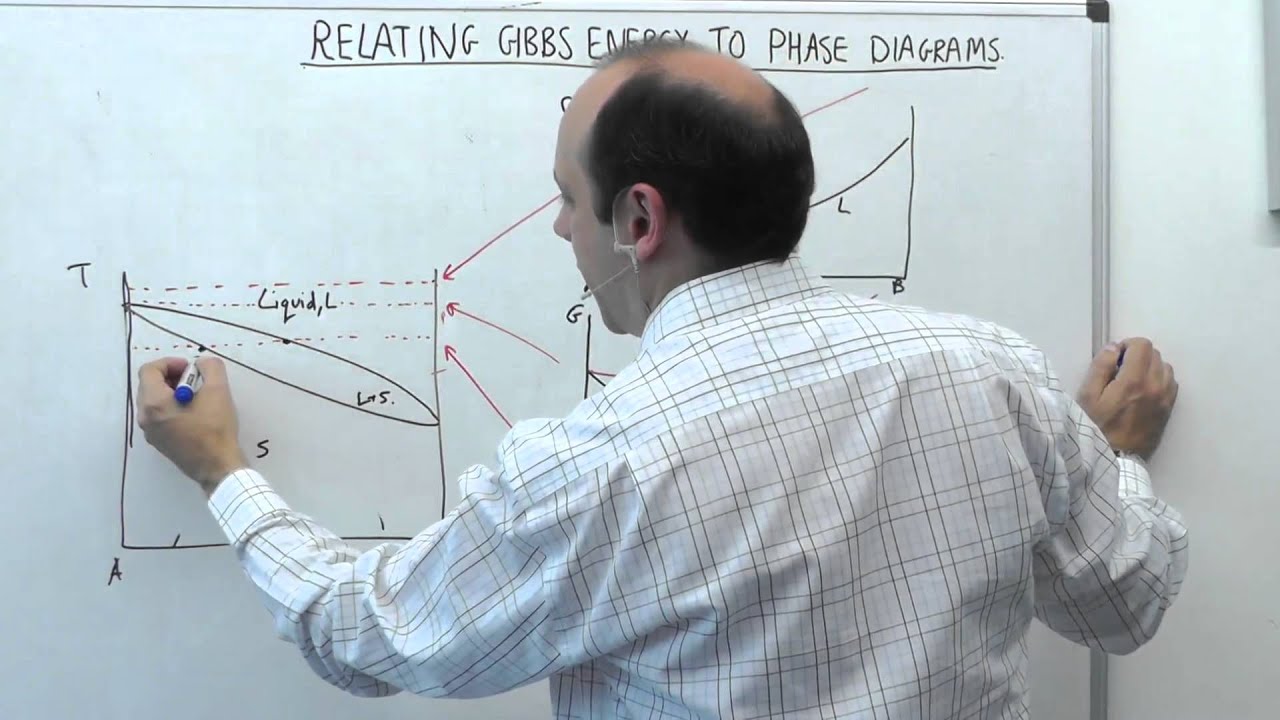
5.2 | MSE104 - Gibbs Energy Curves
Показать описание
Segment 2 of lecture 5. Relating Gibbs Energy Curves to Phase Diagrams - i.e. the common tangent construction.
Lecturer: Dr David Dye.
Licence: Creative Commons
Department of Materials, Imperial College, London, UK
Lecturer: Dr David Dye.
Licence: Creative Commons
Department of Materials, Imperial College, London, UK
5.2 | MSE104 - Gibbs Energy Curves
5.1 | MSE104 - Thermodynamics of Solutions
Phase Diagrams | Gibbs Phase Rule (w/ 5 Examples)
Surface Thermodynamics
Gibbs Phase Rule
Thermodynamics Part 5 Calculating Gibbs Free Energy
Lecture 5: Phase Diagrams Lowest Energy Phase Wins!!
Gibbs Energy Curves
Lecture 5: Drawing Free Energy Curves at Isotherms in a Binary Phase Diagram
Gibbs Free Energy
Physical Chemistry for the Life Sciences (2nd Ed) - Chapter 5 - Gibbs & Nernst Equations
1.1 | MSE104 - Introduction to Phases
2.1 | MSE104 - Binary Phase Diagrams
9.1 | MSE104 Non-equilibrium cooling of steels
Gibbs Free Energy Change for Non-Ideal Solution
Phase Rule - Two component system
2.3 | MSE104 - Calculating phase fractions (lever rule)
Gibbs Free energy (dG = VdP - SdT); how it's derived and why we need it
1.8: Binary phase diagrams of systems with limited solubility
PCE71 Excess Gibbs Models and 1-parameter Margules Equation
Gibbs Free Energy
ph12c lecture13 Gibbs free energy
1.5: Binary phase diagrams of systems with full solubility
The thermodynamics of mixing
Комментарии
 0:26:14
0:26:14
 0:48:08
0:48:08
 0:15:08
0:15:08
 0:05:14
0:05:14
 0:04:41
0:04:41
 0:15:11
0:15:11
 0:05:16
0:05:16
 0:26:14
0:26:14
 0:05:33
0:05:33
 0:50:17
0:50:17
 0:19:42
0:19:42
 0:19:27
0:19:27
 0:17:57
0:17:57
 0:28:47
0:28:47
 0:04:15
0:04:15
 0:07:17
0:07:17
 0:12:12
0:12:12
 0:06:23
0:06:23
 0:07:25
0:07:25
 0:10:47
0:10:47
 0:45:48
0:45:48
 1:25:08
1:25:08
 0:09:54
0:09:54
 0:11:07
0:11:07