filmov
tv
Gibbs Free Energy

Показать описание
This lecture is about gibbs free energy in chemistry. I will teach you gibbs free energy in the most easy way. You will also learn numerical problems and mcqs of Gibbs free energy. Gibbs free energy explains whether a particular reaction is spontaneous or not. Remember that Gibbs free energy is equal to enthalpy minus product of temperature and entropy.
To learn more about Gibbs free energy, watch h this lecture till the end.
#gibbsfreeenergy
#thermodynamics
#thermochemistry
#Najamacademy
Join this channel to get access to perks:
To learn more about Gibbs free energy, watch h this lecture till the end.
#gibbsfreeenergy
#thermodynamics
#thermochemistry
#Najamacademy
Join this channel to get access to perks:
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Using Gibbs Free Energy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
16. Thermodynamics: Gibbs Free Energy and Entropy
Gibbs Free Energy
Gibbs Free Energy
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
Up board class 12chemistry chapter 3 numerical trick ।educator coach ।
Gibbs Free Energy (A-Level Chemistry)
18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemis...
Gibbs free energy and spontaneous reactions | Biology | Khan Academy
18.5 Gibbs Free Energy and the Equilibrium Constant
Entropy: Embrace the Chaos! Crash Course Chemistry #20
Gibbs Free Energy
Gibbs Free energy change 1
Gibbs Free Energy, Entropy, Thermochemistry Question, Percent Composition, Bohr's Atomic Model
18.5 Gibbs Free Energy and the Equilibrium Constant | General Chemistry
Lecture 03, concept 10: Helmholtz vs. Gibbs free energy
Gibbs Free Energy, Entropy, and Enthalpy
Gibbs Free Energy
17.1 Equilibrium and Gibbs free energy (HL)
Quick revision - Entropy and Gibbs Free Energy
Gibbs Free Energy and Calculations
Комментарии
 0:08:12
0:08:12
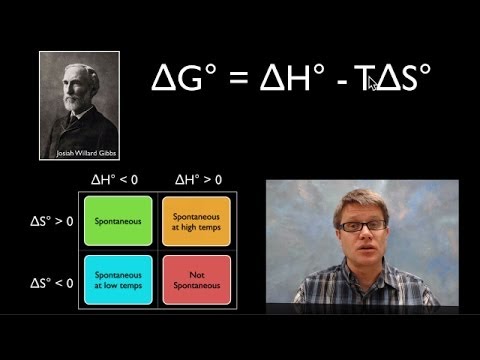 0:07:57
0:07:57
 0:44:45
0:44:45
 0:05:39
0:05:39
 0:32:01
0:32:01
 0:14:13
0:14:13
 0:05:32
0:05:32
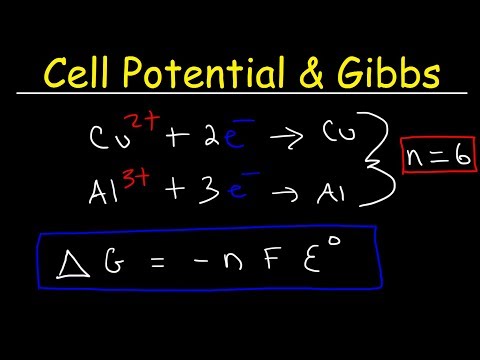 0:11:02
0:11:02
 0:09:41
0:09:41
 0:17:41
0:17:41
 0:32:10
0:32:10
 0:09:17
0:09:17
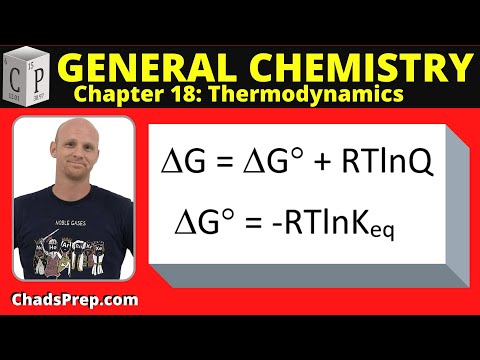 0:11:12
0:11:12
 0:13:41
0:13:41
 0:03:22
0:03:22
 0:11:19
0:11:19
 0:48:43
0:48:43
 0:24:43
0:24:43
 0:05:22
0:05:22
 0:05:33
0:05:33
 0:07:46
0:07:46
 0:05:21
0:05:21
 0:06:43
0:06:43
 0:06:01
0:06:01