filmov
tv
Relationship between Electron Configurations and Valence Electrons

Показать описание
The relationship between electron configurations and valence electrons is crucial in understanding the chemical behavior of elements. Valence electrons are the electrons in the outermost energy level (shell) of an atom, and they are the ones involved in chemical reactions. We will look at Group 1 elements (the alkali metals) as an example.
Join this channel to get full access to Dr. B's chemistry guides:
Group 1 elements include lithium (Li), sodium (Na), potassium (K), and so on, and they all have one valence electron. To understand why, let's look at their electron configurations:
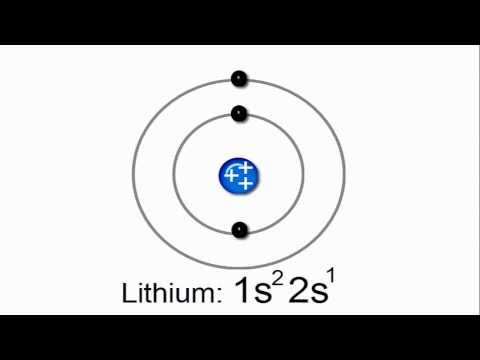
Lithium (Li): Atomic number 3. Its electron configuration is 1s² 2s¹. In this configuration, the 1s² part represents the inner electron shells (core electrons), while the 2s¹ part represents the valence electron in the outermost shell.
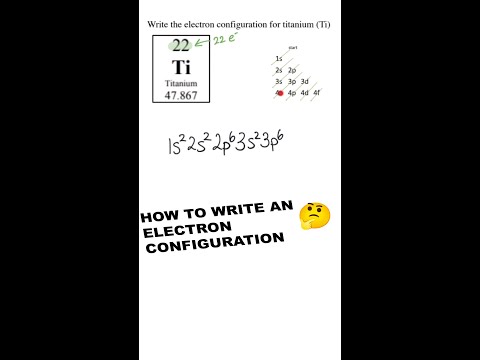
Sodium (Na): Atomic number 11. Its electron configuration is 1s² 2s² 2p⁶ 3s¹. Again, the 1s² and 2s² 2p⁶ represent the core electrons, and the 3s¹ represents the valence electron.
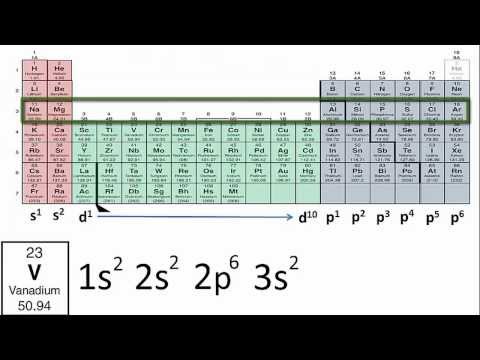
Potassium (K): Atomic number 19. Its electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. Similar to sodium, the 4s¹ electron is the valence electron.
In each of these examples, the valence electron is found in the outermost energy level, which is also the highest-numbered energy level for that element. The number of valence electrons in Group 1 elements is always 1, regardless of the specific element in the group. This is because they all belong to the same group, and elements in the same group have similar electron configurations due to their position in the periodic table.
The number of valence electrons is critical in determining an element's chemical reactivity. Elements in Group 1 tend to be highly reactive because they have only one valence electron, and they strive to lose that electron to achieve a stable, full outer electron shell (similar to the noble gas configuration). This tendency to lose one electron makes them more likely to form positive ions (cations) when they react with other elements, which is a characteristic feature of the alkali metals in Group 1.
Join this channel to get full access to Dr. B's chemistry guides:
Group 1 elements include lithium (Li), sodium (Na), potassium (K), and so on, and they all have one valence electron. To understand why, let's look at their electron configurations:
Lithium (Li): Atomic number 3. Its electron configuration is 1s² 2s¹. In this configuration, the 1s² part represents the inner electron shells (core electrons), while the 2s¹ part represents the valence electron in the outermost shell.
Sodium (Na): Atomic number 11. Its electron configuration is 1s² 2s² 2p⁶ 3s¹. Again, the 1s² and 2s² 2p⁶ represent the core electrons, and the 3s¹ represents the valence electron.
Potassium (K): Atomic number 19. Its electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. Similar to sodium, the 4s¹ electron is the valence electron.
In each of these examples, the valence electron is found in the outermost energy level, which is also the highest-numbered energy level for that element. The number of valence electrons in Group 1 elements is always 1, regardless of the specific element in the group. This is because they all belong to the same group, and elements in the same group have similar electron configurations due to their position in the periodic table.
The number of valence electrons is critical in determining an element's chemical reactivity. Elements in Group 1 tend to be highly reactive because they have only one valence electron, and they strive to lose that electron to achieve a stable, full outer electron shell (similar to the noble gas configuration). This tendency to lose one electron makes them more likely to form positive ions (cations) when they react with other elements, which is a characteristic feature of the alkali metals in Group 1.
Комментарии
 0:10:19
0:10:19
 0:04:17
0:04:17
 0:08:42
0:08:42
 0:06:24
0:06:24
 0:07:23
0:07:23
 0:04:59
0:04:59
 0:12:12
0:12:12
 0:04:52
0:04:52
 1:07:48
1:07:48
 0:11:05
0:11:05
 0:10:17
0:10:17
 0:01:00
0:01:00
 0:03:25
0:03:25
 0:11:19
0:11:19
 0:12:16
0:12:16
 0:03:56
0:03:56
 0:21:44
0:21:44
 0:03:44
0:03:44
 0:01:46
0:01:46
 0:04:28
0:04:28
 0:05:08
0:05:08
 0:14:39
0:14:39
 0:17:24
0:17:24
 0:04:14
0:04:14