filmov
tv
Introduction to electron configurations | AP Chemistry | Khan Academy

Показать описание
Keep going! Check out the next lesson and practice what you’re learning:
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Electron Configuration - Basic introduction
Introduction to electron configurations | AP Chemistry | Khan Academy
Introduction to Electron Configurations
Quantum Numbers, Atomic Orbitals, and Electron Configurations
How to Write the Electron Configuration for an Element in Each Block
Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchool
Electron Configuration
Writing Electron Configurations Using Only the Periodic Table
Electron Configuration [IB Chemistry SL/HL]
Introduction to Electron Configurations
Introduction to electron configurations - Real Chemistry
Introduction to Electron Configurations
The Electron: Crash Course Chemistry #5
Introduction to Electron Configurations
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Basic Introduction of electronic Configuration
Electron Configurations --- An Introduction
Electron Configuration - Electron Subshells - Suborbitals - s, p, d, f - Orbitals - Chemistry
Inside Atoms: Electron Shells and Valence Electron
Electron configurations with the periodic table | Chemistry | Khan Academy
Introduction to Electron Configurations
Introduction to Electron Configurations - Periodic Properties
Introduction to Electron Configurations and the Electron Configurations for Mg
Комментарии
 0:10:19
0:10:19
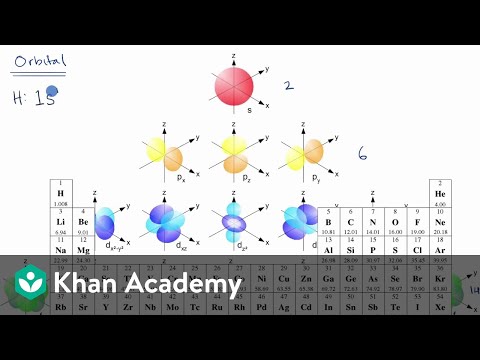 0:05:08
0:05:08
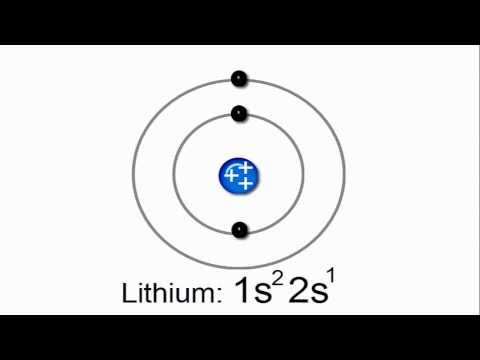 0:01:46
0:01:46
 0:08:42
0:08:42
 0:07:23
0:07:23
 0:04:59
0:04:59
 0:10:17
0:10:17
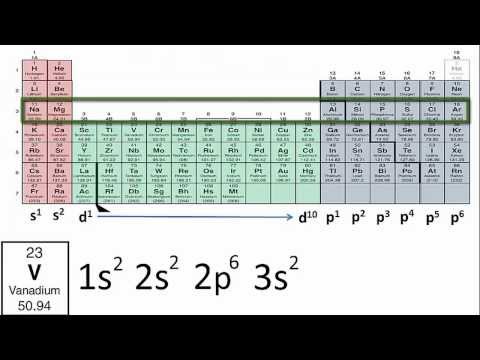 0:04:52
0:04:52
 0:11:05
0:11:05
 0:05:14
0:05:14
 0:09:29
0:09:29
 0:16:56
0:16:56
 0:12:48
0:12:48
 0:34:03
0:34:03
 0:12:12
0:12:12
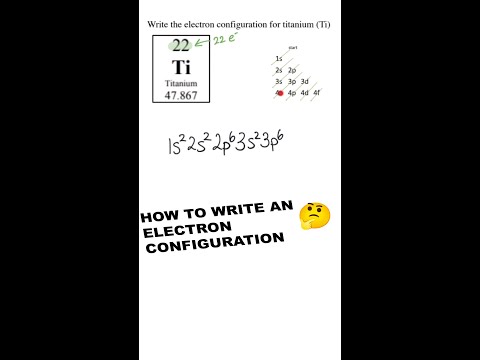 0:01:00
0:01:00
 0:07:28
0:07:28
 0:06:00
0:06:00
 0:20:21
0:20:21
 0:03:25
0:03:25
 0:14:39
0:14:39
 0:11:39
0:11:39
 0:10:22
0:10:22
 0:04:17
0:04:17